To the Editor:
Amyloidosis is an uncommon disease produced by the deposition of fibrillar material that precipitates in the body tissues. The most commonly affected organs are: the kidneys (50%), heart (40%-50%), and peripheral nerves (25%), although it can affect any organ.1 This disease implies a poor prognosis, with 80% mortality two years after diagnosis, despite treatment.2
Here we present two cases of primary amyloidosis that appeared initially in the form of heart failure (HF), hypotension, and progressive renal failure (RF): an uncommon form of evolution for this disease.
Both cases were female patients (aged 58 and 57 years) who sought emergency treatment due to symptoms of HF: one with right HF and the other with left HF. Both patients also had hypotension and mild oedema upon physical examination, and an initial laboratory analysis revealed previously undiagnosed RF (Table 1) with conserved diuresis. In both cases, an electrocardiogram revealed low-voltage sinus rhythm. Given the state of hypotension and signs of heart failure, both patients underwent electrocardiography that revealed a restrictive pattern of mitral filling, suggestive of hypertrophic cardiomyopathy (as opposed to restrictive). Simultaneously, we performed an analysis of RF, with ultrasound images revealing the kidneys to be morphologically normal. We determined the protein/creatinine (Cr) ratio, which was 2500mg/g Cr in one patient, and almost normal (66mg/g Cr) in the other patient. Given the finding in both cases of normochromic normocytic anaemia, with elevated sedimentation rates and renal failure, negative sediment results, and a restrictive pattern in ultrasound analyses, we established the preliminary diagnosis of a systemic infiltrative pathology such as amyloidosis, which led to tests for immunoglobulins and light chains in blood and urine samples. The results from these tests revealed a monoclonal gammapathy. We administered myelograms that confirmed the diagnosis of multiple myeloma (in the first case, lambda IgA, and in the second, lambda IgG), with 24% and 22%-40% infiltration, respectively. Given the suspicion of associated amyloidosis, we performed biopsies of the rectal submucosa, which were positive for Congo red stain tests and birefringence, confirming the diagnosis of AL amyloidosis. Both patients started treatment with bortezomib and prednisone, but the first patient experienced a poor evolution, requiring renal replacement therapy followed by the development of acute pulmonary oedema with cardiogenic shock that was not improved by vasoactive drugs, followed by death after a few weeks.
Amyloidosis is a systemic disease that affects several organs at the moment of diagnosis. In primary amyloidosis, the protein deposits include light chains from the immunoglobulins produced by clonal proliferation of plasma cells, primarily due to multiple myeloma. Asymptomatic deposits of amyloid material can be observed in 30% of patients,1,2 and 10%-15% develop symptomatic AL amyloidosis.3,4 Both myeloma and amyloidosis can produce renal manifestations: renal involvement in multiple myeloma is multi-factorial, although the most common finding is referred to as “myeloma kidney” (60% of cases), which is characterised by tubulo-interstitial damage that is clinically expressed as acute or chronic RF due to tubular light chain precipitation. The majority of patients progress with proteinuria, which is non-selective in 90% of cases, and 25% of these patients develop nephrotic syndrome. Patients with vascular involvement develop only mild proteinuria, but RF continues to progress due to the decrease in renal flow.
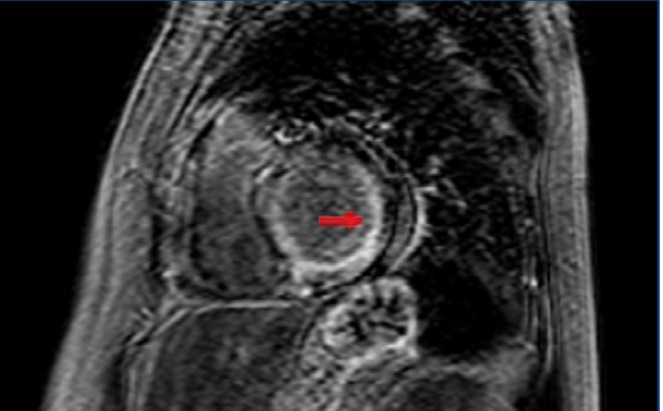
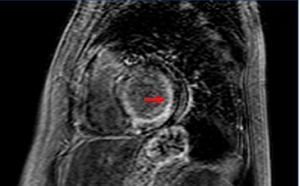
The heart is another organ often implicated in amyloidosis (figure 1). Cardiac involvement can be observed in 50% of patients with AL amyloidosis. Cardiac involvement should be suspected in patients with primarily right HF symptoms, with conserved systolic function and diastolic dysfunction.3 Pulmonary oedema is not a common complication. Established myocardial damage is evaluated by determining troponin and atrial natriuretic peptide levels, which can be used to monitor response to treatment. In order to confirm the diagnosis of amyloidosis, a positive biopsy test must be produced using Congo red stain in affected tissue, and if cardiac involvement is suspected, a positive cardiac imaging test (echocardiogram or magnetic resonance), or even an endomyocardial biopsy, which is a relatively safe procedure when performed by experienced technicians, is needed.1,5
There are two critical components that affect the survival of patients with amyloidosis: cardiac involvement and response to treatment.3 Patients with cardiac involvement have a mean survival of 1.1 years after diagnosis, and a survival less than 6 months if treatment is not provided once the first symptoms of HF are recognised,1,3,5,6 especially if the signs of heart failure persist when the diagnosis is confirmed. Even when the primary manifestation of the disease is in another organ system, cardiac involvement implies a worse prognosis. Only in select cases of isolated cardiac involvement have heart transplants followed by bone marrow transplants been attempted with positive results.4,6
To conclude, the primary manifestations of AL amyloidosis are in the kidneys and heart. Given a hypotensive patient with progressively deteriorating renal function and ultrasound imaging results indicative of infiltrative cardiomyopathy, vascular amyloidosis should immediately be suspected as a possible diagnosis, and an aetiological examination should be started to rule out associated myeloma, in addition to completing the diagnosis of amyloidosis with a tissue sample analysis. The approach for this disease must be multi-disciplinary, evaluating available diagnostic techniques and treatments under a consensus model. In the first of our two cases, the disease expanded aggressively in its severe form, involving both organs and the vascular system, producing an atrocious prognosis with severely limited treatment options. In the second case, it may have been that the bortezomib was able to supress the associated amyloidosis, given its positive results in treating myeloma, or at least could facilitate the consideration of bone marrow transplantation in patients with a partial response to treatment.
Conflicts of interest
The authors state that they have no potential conflicts of interest related to the content of this article.
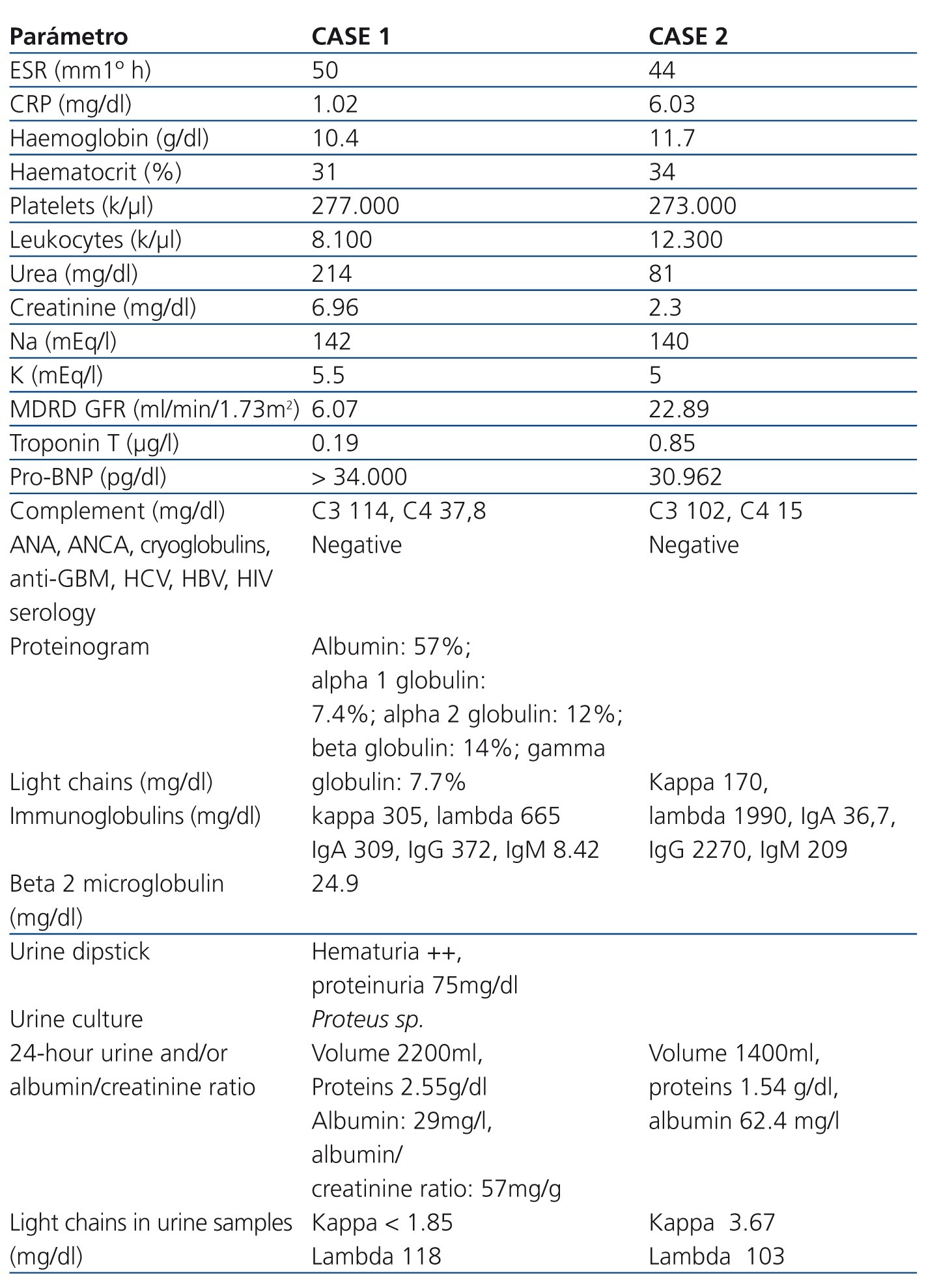
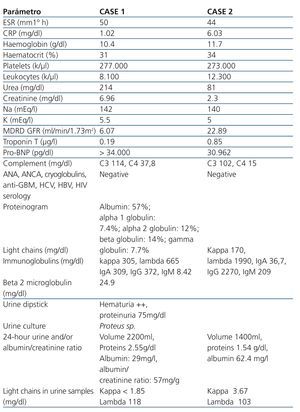
Table 1. Laboratory parameters of the two cases upon hospitalisation
Figure 1. Cardiac magnetic resonance. Delayed gadolinium enhancement in the subendocardium