To the Editors:
The appearance of Henoch-Schönlein purpura (HSP) following kidney transplantation is an uncommon occurrence that has mostly been described as a recurrence of a previous condition.1,2 Very few cases of de novo post-transplant HSP have been described.3 Relapse of glomerulopathy is the third-leading cause of graft loss 10 years after transplantation.4 We report the case of a patient with a history of macrohaematuria and glomerulopathy, who developed vascular purpura, abdominal pain, and urinary alterations 2 years after receiving a kidney transplant. We discuss the indications for a renal biopsy, its results, treatment, and patient evolution.
CASE REPORT
Our patient was a 61-year old white male with a history of macrohaematuria and arterial hypertension after an oropharyngeal infection at 21 years of age. A renal biopsy (RB) was taken at 31 years of age in Australia (unknown result). In December 2006, at 57 years of age, the patient was started on peritoneal dialysis.
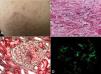
In April 2008, the patient received a kidney transplant from a cadaveric donor. The patient’s creatininaemia upon discharge was 0.8mg/dl, with no proteinuria. Immunosuppression therapy included cyclosporine, mycophenolate mofetil (MMF), and prednisone. 26 months after transplantation, the patient showed dry cough, feverish symptoms, abdominal distension, and pain. Prior to hospitalisation, we observed violate erythematous papullae on the legs and buttocks. An examination revealed a congestive pharynx. The violet coloured erythematous papullae were 1-3mm in size, and did not disappear with compression of the thighs, buttocks, or feet (Figure 1 A). Palpation of the epigastric area produced pain. The transplanted kidney was without pain or increased size.
Laboratory results are summarised in Table 1. Serology tests were negative for hepatitis B and C viruses, human immunodeficiency virus, anti-nuclear antibodies, and anti-neutrophil cytoplasmic antibodies. Complement was normal, and blood was found in the faeces using immunological methods.
The clinical presentation of signs and symptoms suggested HSP. We performed a skin biopsy (Figure 1 B), with negative immunofluorescence results. We observed a progressive increase in proteinuria, which led us to take a renal biopsy that revealed intra- and extra-capillary proliferative glomerulonephritis with cellular and fibrocellular crescents, mostly segmental in nature (Figure 1 C), with 14 of 25 glomeruli affected. We also observed foci of necrosis and leukocytoclasia (Figure 1 D). Mesangial and pericapillary deposits were primarily IgA.
In accordance with nationally recommended treatment protocols (www.nefroprevencion.org.uy), we started treatment with boluses of methylprednisolone at 1g/d for three days and cyclophosphamide at 15mg/k for 6 months, continuing with prednisone at 1mg/k. We maintained cyclosporine treatment at 2mg/k/d and suspended treatment with MMF. The patient’s evolution is summarised in Table 1.
Approximately 22 months after the appearance of HSP, creatininaemia was 1.21mg%, proteinuria was 0.32g/d, and red blood cells were observed in urine samples.
Moroni et al.1 and Han et al.2 described a risk of recurrence of HSP that varies between 0% and 61%, with a greater rate of recurrence in the case of living donors related to the recipient.
Thervet et al.5 described histological recurrence (IgA deposits) of immunoglobulin A nephropathy (IgAN) one year after transplantation in 69% of individuals.
Shimizu et al.3 described a case of post-transplant extra-capillary IgA glomerulonephritis. The presence of abdominal pain suggested atypical HSP. With the exception of this doubtful diagnosis, there have been no cases described of post-transplant de novo appearance of HSP. In the case described, the absence of purpura prior to transplantation suggests that this is indeed a case of de novo HSP.
In 1995, Araque et al.6 published the first case describing the appearance of HSP in a patient who had not received a transplant, years after being diagnosed with IgAN. In our case, the patient’s previous medical history also suggests IgAN, and the patient currently has HSP.
The recurrence of HSP has a worse prognosis: 50% of patients lose their transplants within 30 months of recurrence, as opposed to 11% in the case of recurrent IgAN.2
HSP in adults is more severe and involves a worse prognosis than in children. The study by Pillebout et al.7 (250 cases) reported a 25% rate of extreme renal failure in adult patients. Initial renal function and level of proteinuria are both markers for poor prognosis, as well as glomerular necrosis, and chronicity elements. Our case showed an important inflammatory component, cellular crescents, and necrosis in more than 50% of glomeruli. Soler et al.8 reported that the renal prognosis worsened in the presence of cellular crescents.
The discordance between clinical and histological results suggests the need for performing a renal biopsy on all adult patients with compromised renal function in the context of HSP.
As regards treatment, immunosuppression following transplantation did not prevent the appearance of HSP. This has been reported in other studies as well.2,9,10
While the treatment strategy for HSP is a source of controversy, small studies have demonstrated a benefit from aggressive treatment protocols in these patients. However, the study published by Pillebout in September 2010,11 after our patient was treated, showed that cyclophosphamide provides no additional benefits. In this study, extra-capillary proliferation ranged between 27% and 37% of patients in each treatment group. Our patient had more severe active lesions. Maintenance therapy was carried out using azathioprine, given the reports of scarce usefulness of MMF in treating IgAN from the medical literature.12,13 We observed a progressive reduction in proteinuria, which currently is below 0.5g/d, with stable renal function.
The notable conclusions from this case report are:
1. The de novo appearance of post-transplant HSP despite the use of immunosuppression therapy.
2. The need for an early histological analysis given the discordance between clinical and pathological findings.
3. The controversy regarding how to treat this condition, taking into account that the evolution of HSP is quite severe in adults, and the fact that more aggressive treatment strategies were recently questioned by the Pillebout group.
Conflicts of interest
The authors state that they have no potential conflicts of interest related to the content of this article.
Table 1. Laboratory results and treatment provided
Figure 1. Skin and kidney histology