To the Editor,
The use of acetazolamide for the treatment of Meniere’s syndrome1,2 is uncommon, since other drugs constitute the first line used in the treatment of this pathology.
Here we present the case of a 70-year old woman whose most relevant background involved Meniere’s syndrome, currently treated with acetazolamide at 250mg/12h with oral potassium supplements.
The patient was referred to us due to severe renal failure (urea: 194mg/dl; C-reactive protein: 8.1mg/dl), with extremely severe metabolic acidosis (venous gas: pH: 7.1, bicarbonate: 6.5mmol/l, PCO2: 18mm Hg, PO2: 69mm Hg) and anuria. Laboratory analyses from a few months prior indicated C-reactive protein of 1mg/dl. The patient had been admitted to the nearest reference hospital 24 hours before as a result of severe diarrhoea, and continued taking the normal treatment. Hydration treatment had been started without diuresis, and the patient was transferred for monitoring. Upon hospitalisation, dehydration signs were present, with a blood pressure of 80/50mm Hg, anuria, and no fever. At this point the patient did not have diarrhoea.
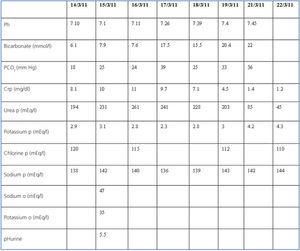
Laboratory analysis revealed acidosis, hypokalaemia (K: 2.9mEq/l) and renal failure similar to that described. We continued to provide intense rehydration therapy, despite which the patient continued with anuria for 24 hours more, with C-reactive protein levels reaching 11mg/dl. Once the volume level was normalised, diuresis started to improve, with clinical and laboratory improvements until reaching a C-reactive protein of 1.2mg/dl upon discharge, with a complete acid-base correction. The evolution of the laboratory values are shown in Table 1.
The patient was discharged with the diagnosis of prerenal acute renal failure due to severe hydrosaline depletion. Metabolic acidosis with a normal anion-gap, in the context of acute renal failure due to the ingestion of acetazolamide and diarrhoea.
Metabolic acidosis is defined as a process in which the blood pH decreases, with the first effect of decreased bicarbonate concentrations followed by the secondary effect of reduced PCO2. Our patient was admitted with acidosis: a pH of 7.1 and a bicarbonate level of 6mEq/l. The acidaemia caused increased ventilation that induced a hypocapnic response that tends to normalise pH. The level of compensation expected can be deduced from the following formula: pCO2 = 1.5 [HCO3] + 8 (± 2). In our patient, the level of compensation was adequate (pCO2: 17mm Hg), and so this was a case of pure metabolic acidosis, with correct respiratory compensation.3,4
The patient’s anion gap upon hospitalisation was normal, near 12. The cases of metabolic acidosis with a normal anion gap (hyperchloremic) suggest that bicarbonate has been replaced by chlorine as it is lost. The most common causes of this type of acidosis are gastrointestinal loss through diarrhoea and renal loss which, in our case, were both caused by acetazolamide.
The patient had low potassium levels, lost through the faeces, and volume depletion that increase the synthesis of renin and aldosterone, which in turn raises the renal secretion rate of potassium, in this case, upon admission, to 2.9mEq/l despite oral supplements. Instead of acidic urine we can observe urine pH of six or higher because the metabolic acidosis and hypokalaemia increase the renal excretion and synthesis of NH4+ and in turn buffers the H+ and increases urine pH. The fractional excretion of sodium tends to be low (<1%-2%) in patients with gastrointestinal losses of HCO3, although this value was high in our patient, without the classic inversion of sodium/potassium in urine samples typical of prerenal conditions due to the combined effects of diarrhoea and diuretics.5,6
In the case that proximal reabsorption of bicarbonate is ineffective due to the use of acetazolamide, which inhibits carbonic anhydrase, bicarbonate losses are produced, such that this molecule escapes from the proximal tubule and reaches the cortical collecting tubule or the distal convoluted tubule, producing reabsorption of sodium and excretion of potassium and hydrogen ions. The low permeability of bicarbonate impedes its reabsorption, which creates a negative potential in the tubular lumen that increases the excretion of potassium and hydrogen ions.
With regard to treatment, the correct approach is to treat the diarrhoea, as well as hydrosaline correction in order to halt the perpetuating stimulus of the renin-angiotensin-aldosterone pathway, with the use of bicarbonate in a continuous perfusion and potassium chloride in order to replace the lost potassium, along with the evident removal of the diuretics. In this manner, our patient responded satisfactorily, with an excellent clinical evolution.
Conflicts of interest
The authors have no conflicts of interest to declare.
Table 1. Evolution of laboratory parameters