Determination of potassium levels in serum is routinely performed in clinical labs, as it is a critical parameter and abnormal potassium concentrations may lead to fatal cardiac consequences.1 An immediate test for determination of blood potassium may contribute to prevent such critical issues, for example, arrhythmias. Therefore we have developed and tested a novel point-of-care (POC) device that provides a simple and highly affordable way of measuring potassium levels in a single drop of blood outside the clinical laboratory.2
We have measured the concentration of potassium in blood of 36 patients suffering from chronic kidney disease (CKD) before and after each dialysis session during three weeks with the POC presented and a reference method following the routine workflow in a clinical laboratory. A total of 705 measurements of potassium have been analyzed and compared using statistical tools. ADVIA Chemistry XPT from Siemens Healthineers was used as the reference system for the potassium measurement, this system measures potassium in plasma with a predilution of 1:33, while the POC device performs a direct measurement of potassium in whole blood with ion-selective electrodes technology.
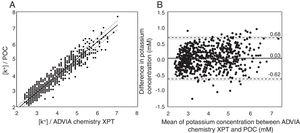
Passing-Bablock regression and Blant-Altman analysis (Fig. 1) confirmed that there is a strong correlation (Pearson correlation coefficient is R2=0.953) between the results obtained by both methodologies with a small bias (0.03) and limits of agreement in -0.62 and 0.68mM. Whether the bias and limits of agreement are adequate or not, it is a clinical rather than a statistical question. As normal potassium levels in blood can vary from 3.5 to 5.5mM a bias of 0.03mM can be considered insignificant from a clinical standpoint. Moreover, the confidence intervals obtained by the POC device (−0.62/0.68mM) are in good agreement with previous validation studies of POC devices3–5 and suitable for the type of situations where a POC device will be used – such as screening for general conditions in the global population or control and monitoring of chronic patients-. The higher contribution to the variability of the technique comes from the precision of the sensor (0.25mM). Other parameters such as the manual calibration or some interferences from blood may contribute to the remaining variability. Another parameter that has to be considered is that the ADVIA Chemistry XPT system measures with indirect ion selective electrodes in a pre-diluted sample, while the POC device measures with direct ISEs in whole blood. Lipemia in serum causes a reduction in the aqueous fraction leading to abnormally lower values, which does not happen in the whole blood measurements. This may be an additional source of variation between the two methodologies.6
(A) Passing–Bablock regression analysis. Dashed lines represent the 95% CIs. POC=1.0769 (95% CI 1.1053/1.0455) ADVIA Chemistry XPT – 0.3231 (95% CI −0.4158/−0.2205), n=694. (B) Comparison of potassium values between the reference technique and the developed potassium sensor by Bland–Altman plots displayed in difference in mM. The solid black line is the average difference, the dashed lines the limits of agreement, and the gray lines the 95% CIs.
This POC device is not conceived to compete with the reference technique in the healthcare facility, but rather to complement the conventional approaches by giving an immediate response when required. Conventional parameters such as sensitivity, limits of detection and linear range are comparable in both techniques. And although the value is less precise, it may have a great impact when triaging life-threatening conditions. Hence, the relative importance of the analytical parameters must be evaluated under different contexts. Thus, the POC device may have a significant impact on the early detection of several medical conditions that will avoid future problems and complications for the patient health and wellbeing, eventually reducing the costs of the treatment.
The POC device meets the requirement for direct analysis since it uses a small amount of blood and no pretreatment is performed although precision is inferior to clinical lab analysis. However, this lower precision is common in POC devices, for example, FDA accepts glucometers with an accuracy of +/−15% for 95% of the results within all the range, and +/−20% for 99%.7 The POC device presents the benefits that this implies, especially the reduction of the time between the sample withdraw from the patient and the given result. This POC device has the particular advantage to work with paper-based sensors, which are suitable for mass-scale production and, due to the materials used, present very low manufacturing cost.
In conclusion, the POC device has demonstrated a good correlation with the reference method, proving that it can be an alternative for specific situations where an immediate response is required. What remains for the future is to validate its utility as an alternative method for specific situations, which depends on whether the reduction of the analysis time can have benefits in the decision making process of the doctors and the quality of life of the patients.
Conflict of interestThe co-authors (Andrade FJ, Blondeau P, Maceira A and Novell M) disclose a potential conflict of interest as they are co-founders of CreatSens Health, SL which intends to commercialize blood bio-sensing technologies. No potential conflicts of interest exists for the rest of the authors.
The authors would like to acknowledge the financial support from the Spanish ministry of Economy and Competitiveness and European Regional Development Fund (ERDF) (Project CTQ2016-77128-R) as well as the Fundación Renal Iñigo Alvarez de Toledo (FRIAT) and the Fundació Miarnau. The patients involved in the study and the staff from the Dialysis section from Hospital Clínic de Barcelona are also acknowledged for their collaboration and enthusiasm in this study.