Cyanosis nephropathy is an infrequent and not well known condition, so the description of a case should be of interest.
A 49-year-old man attended outpatient clinic for proteinuria. He was diagnosed with tricuspid atresia in his early childhood, treated surgically with classic Glenn intervention (anastomosis of the superior vena cava to the right pulmonary artery) at 20 months of age, and in a second intervention by means of Blalock-Taussig pulmonary systemic fistula left (GORE-TEX® graft prosthesis from the subclavian artery to the homolateral pulmonary artery), which subsequently had to be repaired with an endovascular stent due to stenosis. He had moderate left ventricular dysfunction with chronic cyanosis, with habitual Hb values of 17g/dl and Hct of 60%. Moderate thrombocytopenia and transient ischemic attack 10 years earlier, after which antiaggregation with ASA was replaced by clopidogrel. Regular treatment included clopidogrel, allopurinol and bosentan. In addition, he had periodic phlebotomies until 2 years ago when they were suspended at the discretion of their cardiologists. The patient consulted for a proteinuria of unknown time of evolution. Normal blood pressure with a BMI of 23kg/m2. In the analytic, Cr 0.78mg/dl with estimated glomerular filtration rate by CKD-EPI greater than 90ml/min/1.73m2, Hb 23.8g/dl; Hct of 80.8%; platelets 89,000/ml; uric acid 7.9mg/dl; cholesterol 257mg/dl; systematic urine with minimal microhematuria; MAu/Cru 3371.4mg/g and proteinuria of 6.21g/24h of mixed type. Negative viral serology. Negative immunology tests including complement, ANA, ANCA, anti-DN, anti-phospholipase and anti-PLA2R antibodies. Immunoglobulins and proteinogram were normal. Abdominal ultrasound with kidneys of conserved size and bilateral diffuse hyper echogenicity as the only finding that that was noteworthy.
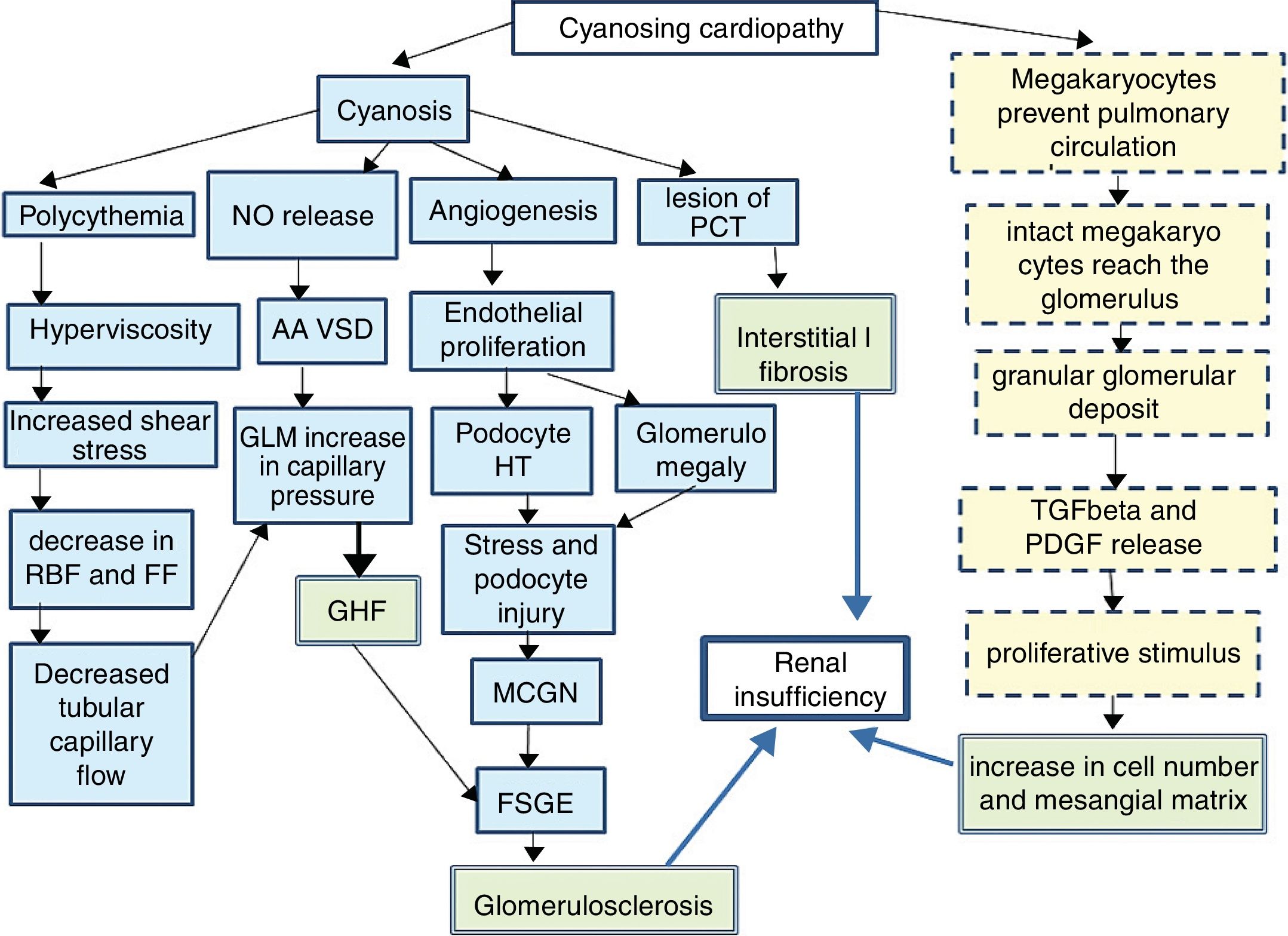
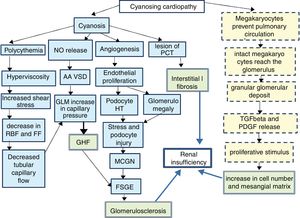
Since 1960,1 it has been known that between 30 and 50% of cyanotic heart diseases can be the cause of a secondary glomerulopathy known as cyanosis nephropathy.2 It is a correlate of cardiorenal syndrome type 2. It is produced trough to different mechanisms (Fig. 1)3–5 initiated by hyperviscosity syndrome secondary to extreme polycythemia and cyanosis, which cause an increase in shear stress by passing a high number of red blood cells through the capillary unit in the glomerulus. The subsequent decrease in renal blood flow and filtration fraction induce hypoxia, with an angiogenic response mediated by the release of nitric oxide, selective vasodilatation of the afferent arteriole and increased capillary pressure, which determines glomerular hyperfiltration. The decrease in peritubular capillary blood flow also contributes to increase in capillary pressure. In parallel, cyanosis triggers an angiogenic stimulus with an increase in the number of capillaries per glomerulus, glomerulomegaly due to endothelial proliferation and an increase in the surface area of the glomerular capillary, the podocyte stretches and it is hypertrophied due to stress and there is a damage that mimics glomerulonephritis due to minimal changes and subsequently to segmental glomerulosclerosis.6
Pathophysiology of cyanotic nephropathy.
FF: filtration fraction; RBF: renal blood flow; FSGE: focal and segmental glomerulosclerosis; GLM: glomerulus; MCGN: minimal change glomerulonephritis; GHF: glomerular hyperfiltration; HT: hypertrophy; PDGF: platelet derived growth factor; PCT: proximal convoluted tubule; TGFβ: tumor growth factor β; AA VSD: arteriolar afferent vasodilation.
Continuous line: pathway of vascular damage; broken line: path of proliferative damage; double grid: path of final damage.
It is accepted that the initial damage is in the tubule,7–9 especially in the proximal tubule, as reflected by an increase in urinary N-acetyl glucosamine and α1-microglobulin that usually occurs in the first decade of life and continue in the next decade with a glomerular damage (albuminuria, proteinuria and decrease in glomerular filtration). As the mechanism of compensating glomerular hyperfiltration is exhausted, a glomerular hyalinosis develops with glomerulosclerosis and interstitial fibrosis that will condition the midterm prognosis. A different injury is distinguished in small vessels resulting from capillary dilation, thickening or endothelial destruction, glomerulosclerosis and periglomerular fibrosis mediated by a non-vascular proliferative mechanism, resulting from the shunt in the pulmonary circulation. Under normal conditions, after being released from the spleen and bone marrow, a population of megakaryocytes are retained in the interstitium and in the pulmonary vasculature5,10,11 where they disaggregate forming active platelets at a rate of 106/h. However in conditions of right/left shunt this step is avoided and the megakaryocytes pass intact to the systemic circulation making possible that their alpha granules, rich in PDGF and in TGFβ, reach the renal glomerulus and be released, producing 2 effects. A peripheral thrombocytopenia that indirectly quantifies the degree of shunt (the greater thrombocytopenia, the greater shunt) and an increase of proliferative platelet factors that will cause an increase in mesangial juxtaglomerular cells, mesangial matrix and focal interstitial fibrosis.
The hematocrit level, the delay until shunt surgery and a platelet level of less than 250,000/ml are risk factors for the appearance of nephropathy.3,12,13
The treatment consists of reducing surgical waiting times, periodic phlebotomies and inhibition of the renin–angiotensin–aldosterone axis with ACE inhibitors.14
Renal prognosis is unfavorable if the cause is not resolved. In our case, we began treatment with statins and ACEIs, and periodic phlebotomies were again recommended without sufficient follow-up to evaluate the response at the present time.
Please cite this article as: Ortega-Díaz M, Puerta Carretero M, Corchete E, Martín Navarro JA, Jaldo MT, Albalate M, et al. Nefropatía por cianosis. A propósito de un caso. Nefrologia. 2019;39:96–98.