AA amyloidosis (AAA) is one of most severe complications of chronic inflammatory and infectious diseases and may induce nephrotic syndrome.1 Its association with Crohn's disease has been regularly reported, but amyloidosis complicating ulcerative colitis (UC) has been described only exceptionally.2
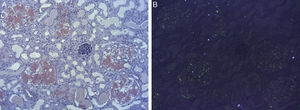
We report a case of a 58-year-old woman who was admitted with anasarca and nephrotic syndrome (NS). Relevant medical history included UC, gangrenous pyoderma and a tuberculous empyema. In 2002, a NS was identified and a renal biopsy displayed the diagnosis of AAA. In addition to treatment with 5-aminosalicylic-acid, a trial of colchicine was implemented but had to be stopped due to diarrhea. Renal function (RF) was sustainably preserved and the proteinuria spontaneously remitted. Throughout time she had long-lasting, moderate gastrointestinal symptoms with evidence of mild endoscopic inflammation. In 2015 she developed once more disabling proteinuria with normal RF and a diagnostic workup corroborated the clinical impression of recurrence of her amyloidosis (Table 1). An endoscopic review exhibited mild disease and, in addition to messalazine and prednisone, she was started on azathioprine, which had to be suspended due to pancreatitis. Therapy with an angiotensin-converting enzyme inhibitor was initiated but not tolerated; albumin-plus-furosemide infusion led merely to temporary improvement. A decision to deteriorate RF to decrease proteinuria was attempted: indomethacin led to acute renal dysfunction but didn’t significantly improved proteinuria. Hemodialysis was started but had to be interrupted due to hemodynamic instability. After a multidisciplinary consultation, she underwent a bilateral nephrectomy. Post-operatively, serum albumin and postural symptoms gradually improved with subsequent better hemodialysis tolerance. Histology revealed extensive amyloid deposits (Fig. 1) and immunohistochemical staining was positive for AA amyloid. Nutritional and functional status gradually improved.
Laboratory tests results.
| Result | Reference range | |
|---|---|---|
| Hemoglobin (g/dL) | 9.7 | 12–16 |
| Leukocytes (103/μL) | 9.3 | 4–11 |
| Platelets (103/μL) | 183 | 150–400 |
| C-reactive protein (mg/L/sedimentation rate 1st h) | 24/127 | <5/0–30 |
| Total protein/albumin (g/dL) | 4.4/1.2 | 6.4–8.3/3.5–5.0 |
| AST/ALT/LDH (U/L)a | 11/18/205 | 5–34/<55/110–220 |
| Fasting glucose/total bilirubin (mg/dL) | 83/0.7 | 60–110/0.2–1.2 |
| Total/LDL/HDL cholesterol (mg/dL) | 270/169/40 | <200/<100/≥60 |
| Urea/creatinine (mg/dL) | 52/0.7 | 21–43/0.6–1.1 |
| Urinalysis/24 hours protein (mg/day) | 4+ proteinuria/19538 | 0+/<300 |
| Serum immunoelectrophoresis/serum kappa-lambda ratio | Absence of monoclonal gammopathy/normal | |
| Urinary electrophoresis/urinary immunoelectrophoresis | Albumin=46% and gamma globulin=14%/no band suggestive of monoclonality | |
| Tests for hepatitis B and C, HIVb, and syphilis | Negative | |
| ANA, anti-dsDNA and ANCAc antibodies; C3 and C4 complement levels | Normal | |
| Serum amyloide A (mg/L) | 79.4 | <7 |
AAA in the setting of UC is a rarity and typically, severe gastrointestinal illness precedes the development of amyloidosis,3 however this was not the case in our patient, who had a long standing, though mild disease. We speculate that the ongoing activity and the prolonged duration may have had a role; nevertheless, an additional contribution from the tuberculosis cannot be ruled out.
Another peculiar aspect is the spontaneous remission of the patient's NS. Improvement of amyloidosis manifestations is extensively documented in the context of remission of the underlying disease, but spontaneous regression is extremely unlikely.4
Patients with large proteinuria due to AAA can experience a life-threatening cachexic state with massive edema, respiratory distress and infectious complications, along with a poor functional status and a reduced quality-of-life. Up to the present, AAA has no specific treatment and despite the development of novel therapies (eprodisate)5 targeting the formation/stability of fibrils, the current available approach is to treat the underlying condition and thereby reduce the amyloid production. Nevertheless, this goal is not always achieved and the management of proteinuria can be challenging, resorting, in intractable cases like ours, to renal ablation. Medical ablation, with non-steroidal anti-inflammatory drugs6 has been attempted; bilateral renal artery embolization is an alternative, although frequently complicated by a ‘post-infarction syndrome’.7 Recently, bilateral ureteral ligation8 has been described, but the experience is limited and can induce persistent loin pain and ascending infections. Surgical nephrectomy is another option: although laparoscopic surgery is a less invasive procedure than an open nephrectomy, it precludes the chance for peritoneal dialysis and, consequently, we elected a bilateral nephrectomy by lombotomy.
The histological examination of the kidneys revealed extensive presence of AA amyloid deposits comprising the glomeruli, the blood vessels and the interstitium, an infrequent finding in the review by Hopfer et al.,9 who reported interstitium involvement in only 5.9%.
AAA has a major impact in patient's quality of life: Esteve et al.10 reported that in 1/3 of the patients, dialysis was not performed due to their poor clinical condition and disturbing quality of life. Bilateral nephrectomy in our patient, led to an improvement in hemodynamic stability allowing hemodialysis to be accomplished. Additionally, her functional and nutritional status enhanced gradually.
Radical bilateral nephrectomy may be considered in seriously ill patients with nephrotic syndrome and disabling complications of proteinuria.
Conflicts of interestThe authors declare that they have no conflict of interest.