Quinoline-3-carboximide compounds, such as paquinimod, which targets the protein S100A9, have demonstrated efficacy in treating autoimmune diseases. S100A9, in association with S100A8, forms the heterodimer S100A8/S100A9, known as calprotectin; that has been shown to be upregulated in numerous inflammatory disorders. We had previously demonstrated protection from glomerular disease in S100A9-deficient mice. The aim of this study was to assess the efficacy of paquinimod in the prevention and treatment of experimental glomerulonephritis.
MethodsNephrotoxic nephritis (NTN) was induced in C57BL/6 mice according to our standard protocol. Mice were treated with different doses of paquinimod either at disease induction (prevention group) or two days following induction (therapeutic group) and sacrificed 8 days following induction. Disease was assessed histologically (number of glomerular crescents, degree of glomerular thrombosis, number of infiltrating leucocytes and calprotectin expression) and biochemically (serum creatinine and urea levels, and urinary levels of protein).
ResultsNeither treatment with low (0.5mg/kg) or high (25mg/kg) doses of paquinimod, given preventatively or therapeutically, led to disease attenuation, as assessed by biochemical or histological parameters. Additionally, we found trends for an increase in renal glomerular calprotectin expression in the high dose groups, suggesting a possible feedback regulation of calprotectin expression.
ConclusionsOur results show that paquinimod does not successfully prevent or treat mice with NTN. Other models of immune-mediated glomerulonephritis need to be tested to investigate the therapeutic potential of this compound in renal disease.
Los compuestos relacionados con la 3-carboxamina-quinolina han demostrado eficacia en el tratamiento de enfermedades autoinmunes. Uno de estos compuestos, el paquinimod, ejerce su función a través del targeting de la proteína S100A9. La proteína S100A9, en asociación con S100A8, forma el heterodímero S100A8/S100A9, conocido como calprotectina, que se ha demostrado que está elevada en variadas enfermedades inflamatorias. Habíamos demostrado anteriormente la protección contra la enfermedad glomerular en ratones deficientes de S100A9. El objetivo de este estudio fue evaluar la eficacia de paquinimod en la prevención y el tratamiento de la glomerulonefritis experimental.
MétodosLa nefritis nefrotóxica (NTN) se indujo en ratones C57BL/6 de acuerdo con el protocolo estándar. Los ratones fueron tratados con diferentes dosis de paquinimod en el momento de la inducción de la enfermedad (grupo de prevención) o 2 días después de la inducción (grupo terapéutico)y se sacrificaron 8 días después de la inducción. La enfermedad se evaluó histológicamente (número de semilunas glomerulares, el grado de trombosis glomerular, número de leucocitos infiltrantes y de expresión de calprotectina) y bioquímico (niveles séricos de creatinina y urea, y los niveles urinarios de proteínas).
ResultadosNinguno de los tratamientos con bajas (0,5mg/kg) o altas (25mg/kg) dosis de paquinimod, administrados preventiva o terapéuticamente, resultaron en la atenuación de la enfermedad,según los parámetros bioquímicos o histológicos. Además, encontramos una tendencia al aumento en la expresión renal de la calprotectina glomerular en los grupos con dosis altas, lo que indica una posible regulación de la retroalimentación de la expresión de calprotectina.
ConclusionesNuestros resultados muestran que el paquinimod no puede prevenir ni tratar con éxito la NTN en los ratones. Otros modelos de glomerulonefritis inmunomediadas necesitan ser testados para investigar el potencial terapéutico de este compuesto en la enfermedad renal.
Paquinimod belongs to the class of quinoline-3-carboximide derivatives, compounds that have demonstrated efficacy in treating autoimmune diseases in both humans and mice.1–5 Linomide, the first compound of this class, was effective in treatment of multiple sclerosis (MS) in phase II studies; however, cardiovascular toxicity during phase III studies led to premature trial termination.6,7 Subsequently, two new compounds with improved safety profiles and increased potency were investigated. The first, Laquinimod exhibited clinical efficacy as well as favourable safety in MS patients8–10 and is still being investigated for the treatment of MS. The second, Paquinimod effectively inhibited disease in experimental lupus prone mice, and was well tolerated (in doses up to 3mg/day) in SLE patients with low disease activity, however, at higher doses (up to 6mg/day) systemic adverse effects consisting of myalgias and arthralgias were reported. In addition, transient increases in inflammatory cells and acute phase reactants were seen at all doses, but most marked at the higher dose range.11
The S100A9 protein has been identified as one target molecule of Paquinimod, disrupting binding of this protein to one of its receptors, TLR-4 but not to the advanced glycosylation end product-specific receptor (RAGE).12 In association with S100A8, S100A9 forms the heterodimer S100A8/S100A9, termed calprotectin. Calprotectin is found abundantly in neutrophils, monocytes and early differentiated macrophages and is involved in calcium-dependent signalling, cell differentiation, cell cycle progression and cytoskeleton-membrane interactions.13 Upon phagocyte activation with the inflamed endothelium, calprotectin is secreted, binds to endothelial cells stimulating the secretion of pro-inflammatory cytokines such as interleukin-8 (IL8) and increasing the expression of Intercellular adhesion molecule 1(ICAM-1), involved in the further recruitment of leucocytes, promoting the impairment of endothelial monolayer integrity and inducing apoptosis and necrosis.14–16
Calprotectin has been demonstrated to be upregulated in many inflammatory disorders, such as SLE, RA, idiopathic juvenile arthritis, Kawasaki's disease and AAV.17–22 In patients with active SLE, serum calprotectin levels correlate with disease activity assessed by laboratory and clinical parameters.17 In patients with limited systemic AAV, failure to suppress serum calprotectin whilst on immunosuppressive treatment was associated with subsequent disease relapse, suggesting that calprotectin may be a useful biomarker to predict future flares.22
In animal models of autoimmunity, calprotectin and its TLR4 receptor have been implicated in mediating disease. In accelerated NTN, a model of immune mediated glomerulonephritis, wild-type (WT) mice with glomerulonephritis have increased serum levels of S100A8/A9, while mice deficient in S100A9 (S100A9−/−) or the TLR4 receptor (TLR4−/−) are significantly protected from disease.23,24 In a model of autoimmunity in mice overexpressing CD40L, S100A8/S100A9 was critical for the induction of dermatitis and nephritis, as well as the expansion of autoreactive CD8 T-cells, and the development of autoantibodies.25 Together these data suggest that S100A8/A9 blockade could be a promising therapeutic target in human glomerulonephritis including lupus nephritis and ANCA associated glomerulonephritis. In the present study we assessed the efficacy of Paquinimod in the prevention and treatment of murine NTN.
MethodsAnimals, induction of accelerated nephrotoxic nephritis (NTN) and treatment with PaquinimodAnimalsMale C57BL/6 mice were between 8 and 12 weeks of age, and were bred in house. All experiments were performed under the terms of the Animals (Scientific Procedures) Act 1986, under UK Home Office animal welfare licences
Nephrotoxic nephritisSheep anti-mouse nephrotoxic serum (NTS) was generated according to previously published methods.26 Disease was induced by pre-immunising mice sub-cutaneously with sheep IgG (0.2mg) in Complete Freund's Adjunvant (CFA, Sigma). Five days later (day 0), mice were injected intravenously with 200μl of sheep NTS (diluted 1:3 in sterile 0.9% NaCl) and intraperitoneal lipopolysaccharide (LPS, 0.1μg/mouse). Mice were sacrificed 8 days later. Groups of 7–10 mice were used per treatment cohort.
Treatment with different doses of PaquinimodMice were treated with Paquinimod (from Active Biotech AB, Lund, Sweden) dissolved in drinking water available ad libitum, after assessment of the average daily fluid intake of the mice. In the first experiment, the compound was used at dosage of 0.5mg/kg per day starting at day 0 in the treatment group (10 mice: paquinimod), while control mice drank normal water (10 mice: controls). In the second experiment, a dosage of 25mg/kg per day was used, starting at day 0 (prevention group: PaqP, 7 mice), or day 2 (therapeutic group: PaqT, 7 mice) or untreated (Control, 7 mice). Mice were placed in metabolic cages overnight, the day before sacrifice and urine collected.
Assessment of renal injurySerum creatinine, urea, ALT and albumin were measured in the clinical pathology laboratory, Mary Lyon Centre, Medical research Council, Harwell. In all experiments, urine was collected after housing mice in metabolic cages overnight with free access to food and water. Urinary protein was measured by the sulfosalicylic acid method.27
Histology and immunohistochemistryKidneys were removed, fixed in formalin and embedded in paraffin for periodic acid-Schiff (PAS) and immunohistochemical staining. Immunoperoxidase staining for mouse calprotectin and macrophages was performed using a rat anti-mouse calprotectin monoclonal antibody (clone 2B10 at 1.2mg/ml, a gift from Prof Nancy Hogg) and a rat anti-mouse MAC2 (at 1mg/ml), respectively. Renal sections were analysed for the percentages of glomerular crescents (0–100%) and the degree of glomerular thrombosis using a standard scoring system (0=no PAS material, 1=0–25% of glomerulus affected, 2=25–50%, 3=50–75% and 4=75–100%).
Statistical analysisStatistical analysis was performed using GraphPad prism 6.0 (GraphPad Software, San Diego, CA, USA). A Mann–Whitney test was used when comparing 2 groups. When comparing 3 groups, a one-way ANOVA test was used. Results were considered significant when p<0.05.
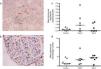
ResultsLow dose preventative Paquinimod treatmentIn the first experiment, a dose of paquinimod of 0.5mg/kg per day was administered from day 0, based on the previously published data demonstrating efficacy in a lupus mouse model.11 All mice tolerated this dose, with no fatalities. Renal function, assessed by serum urea and creatinine, as well as urinary protein excretion, were not significantly different between Paquinmod-treated (n=10) and control (n=10) mice (median serum urea Paquinimod 14.8mmol/l (range 6.9–98.0) vs control 8.2mmol/l (range 5.8–120); median serum creatinine Paquinimod treated 36.6μmol/l (range 25.6–76.3) vs control 34.3μmol/l (range 29.1–86.6); median proteinuria in Paquinimod-treated animals 9.3mg/ml (range 6.3–14.5) vs controls 6.8mg/ml (range 1.1–9.1) (all p=NS)) (Fig. 1a–c).
Renal function assessed by (a) serum creatinine and (b) urea and (c) urinary protein excretion in control and Paquinimod-treated animals; Renal histology assessed by (d) percentage glomerular crescents and (e) glomerular thrombosis score in control and Paquinimod-treated animals. Medians shown. Paquinimod was administered at a dosage of 0.5mg/kg per day.
Similarly, renal histology, revealed no significant differences between the two groups with regards percentages of glomerular crescents (median % crescents paquinimod 8% (range 0–60%) vs controls 10% (range 0–44) or thrombosis scores (median thrombosis score Paquinimod 0.53 (range 0–3.5) vs controls 0.1 (range 0–2.84) (Fig. 1d and e).
High-dose prevention and treatment Paquinimod therapyIn the second series of experiments, the dose of Paquinimod was increased to 25mg/kg per day, based on further published data showing efficacy in the treatment of experimental autoimmune encephalomyelitis.5 After 8 days, 4 mice had died; 2 from the control group, 1 from the prevention group and 1 from the treatment group.
Renal function and proteinuria (Fig. 2a–c) were similar between the three groups(median serum creatinine control 12.1μmol/l (range 10.4–13.7) vs Paq P 17.3μmol/l (range 10.8–55.7) vs Paq T 16.7μmol/l (range 15.2–18.10); median serum urea control 9.5mmol/l (range 9.0–14.1) vs Paq P 35.1mmol/l (range 10.9–148.8) vs Paq T 42.7mmol/l (range 8.1–77.3); Renal histological damage was also similar in all three groups with regards percentages of glomerular crescents (median % crescents control 0% (range 0–20) vs Paq P 4% (range 0–75) vs Paq T 0% (range 0–50) and glomerular thrombosis scores median thrombosis score control 0.1 (range 0–0.49) vs Paq P 0.39 (range 0–0.97) vs Paq T 0.02 (range 0–0.22) (Fig. 2d and e).
Renal function assessed by (a) serum creatinine and (b) urea and (c) urinary protein excretion in controls and animals treated with Paquinimod prophylactically (Paq-P)or therapeutically (Paq-T); Renal histology assessed by (d) percentage glomerular crescents and (e) glomerular thrombosis score in control and Paquinimod-treated animals. Medians shown. Paquinimod was administered at a dosage of 25mg/kg per day.
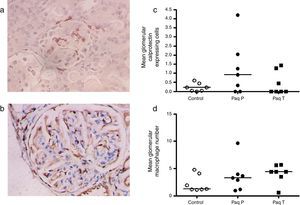
As the drug has direct binding effect in the S100A9 and calprotectin is abundantly expressed in early inflammatory macrophages, we performed an immunoperoxidase staining for calprotectin and macrophages (Fig. 3a and b) and quantification of these showed a trend for increased glomerular calprotectin expression, and a similar trend in glomerular macrophage numbers, (mean glomerular calprotectin cell expression control 0.22cells/glomerulus (SEM 0.1) vs Paq P 1.25 (SEM 0.6) vs Paq T 0.45 (SEM 0.2); mean glomerular macrophage number control 2.2cells/glomerulus (SEM 0.1) vs Paq P 3.6 (SEM 1.0) vs Paq T 4.1 (SEM 0.7)).
Immunohistochemistry for (a) calprotectin expressing cells and (c) macrophages. (b) Quantification of mean calprotectin positive cells per glomerulus and (d) mean glomerular macrophage number in Control, and Paquinimod treated animals (prevention group (Paq P) and therapeutic group (PaqT)). Paquinimod administered at a dosage of 25mg/kg per day.
Previous studies in lupus prone mice,11 experimental autoimmune encephalomyelitis5 and collagenase induced osteoarthritis28 have demonstrated a significant reduction in glomerular, central nervous system and joint inflammation respectively following the use of prophylactic low and high doses of Paquinimod, a compound that inhibits S100A9 but not S100A8, binding to TLR4. We have previously shown that serum S100A8/A9 levels are elevated during NTN and that S100A9 deficiency significantly attenuates disease, in part by inhibiting release of proinflammatory cytokines and chemokines.25 We found no effect of S100A9 deficiency on glomerular neutrophil recruitment, but noted a significant reduction in glomerular macrophage infiltration. In this report, we show that using the same NTN model, different doses of Paquinimod administered at the time of, or following, disease induction were not efficacious in attenuating disease. We found a non-significant trend of increased glomerular calprotectin expression in those animals given the highest dose of Paquinimod, and a similar trend in the number of glomerular macrophages.
Denoric et al.,29 demonstrated that Paquinimod reduced the accumulation of Ly6Chi inflammatory monocytes and eosinophils, but not neutrophils in the omentum or at subcutaneous sites of inflammation. While, in alum-induced inflammation, in which neutrophils were the predominant cell population, Paquinimod failed to reduce the accumulation of inflammatory cells. Therefore our NTN model, which relies on early transient neutrophil recruitment followed by monocyte/macrophage infiltration into the kidney by day 3, may not be easily attenuated by Paquinimod. Another possible explanation for our observations may relate to the findings of Vandal et al.,30 who investigated the role of S100A8 and S100A9 on neutrophil migration into a murine air pouch in response to LPS. They found that LPS led to S100A8, S100A9 and S100A8/A9 in the pouch exudates that preceded the neutrophil accumulation. Blocking S100A8 inhibited neutrophil migration 3h after LPS injection, but blocking S100A9 had no significant effect. It is therefore possible that LPS used in induction of our NTN model resulted in an increase of S100A8 expressing inflammatory cells early on in the course of disease.
In addition, Paquinimod appears to induce a transient increase in levels of inflammatory markers, such as C-reactive protein (CRP) and erythrocyte sedimentation rate, reported in Paquinimod-treated SLE patients.11 These changes were more evident with higher dosages of Paquinimod (4.5mg/day and 6.0mg/day compared to 3.0mg/day). Similar increases in inflammatory markers, were also reported in the first weeks of treatment from clinical trials with Laquinimod.8,9 The mechanisms that cause these changes remain unclear and are being investigated, but if replicated in our NTN model could explain the lack of efficacy observed in a short term animal model. It is noteworthy that the efficacy of Paquinimod in other experimental models have utilised more prolonged time courses compared to ours. Our NTN model may evolve too rapidly to dissect out any transient increase and subsequent reduction in inflammation that may occur with this compound, in contrast to genetic deficiency of S100A9.
ConclusionsIn conclusion, we could not show a benefit from Paquinimod in prevention or treatment of a murine NTN model, despite having previously shown a benefit from complete S100A9 deficiency using the same model. However, due to the transient proinflammatory action of this compound, a murine glomerulonephritis model of longer duration may be necessary to fully test the efficacy of this drug, or the drug may need to be used in combination therapy with other anti-inflammatories such as corticosteroids which could suppress the early inflammatory response.
Authors’ contribution- -
JB: performed animal work, participated in the design of the study, performed the statistical analysis and drafted the manuscript.
- -
RP: performed animal work.
- -
AS: performed animal work, conceived of the study, and participated in its design and coordination.
The authors declare that they have no competing interests.
All authors read and approved the final manuscript.