Anti-neutrophil cytoplasmic antibody-associated vasculitis (AAV) is a multisystemic disease. Despite the improvement in mortality rate since the introduction of immunosuppression, long-term prognosis is still uncertain not only because of the disease activity but also due to treatment associated adverse effects. The neutrophil-to-lymphocyte ratio (NLR) has been demonstrated as an inflammatory marker in multiple settings. In this study, we aimed to investigate the prognostic ability of the NLR in AAV patients.
MethodsWe conducted a retrospective analysis of the clinical records of all adult patients with AVV admitted to the Nephrology and Renal Transplantation Department of Centro Hospitalar Universitário Lisboa Norte from January 2006 to December 2019. NLR was calculated at admission. The outcomes measured were severe infection at 3 months and one-year mortality. The prognostic ability of the NLR was determined using the receiver operating characteristic (ROC) curve. A cut-off value was defined as that with the highest validity. All variables underwent univariate analysis to determine statistically significant factors that may have outcomes. Only variables which significantly differed were used in the multivariate analysis using the logistic regression method.
ResultsWe registered 45 cases of AVV. The mean age at diagnosis was 67.5±12.1 years and 23 patients were male. The mean Birmingham Vasculitis Activity Score (BVAS) at presentation was 26.0±10.4. Twenty-nine patients were ANCA-MPO positive, 7 ANCA-PR3 positive and 9 were considered negative ANCA vasculitis. At admission, mean serum creatinine (SCr) was 4.9±2.5mg/dL, erythrocyte sedimentation rate (ESR) was 76.9±33.8mm/h, hemoglobin was 9.5±1.7g/dL, C-reactive protein was 13.2±5.8mg/dL and NLR was 8.5±6.8. Thirty-five patients were treated with cyclophosphamide, eight patients with rituximab for induction therapy. Twenty patients developed severe infection within the first three months after starting induction immunosuppression. In a multivariate analysis, older age (73.6±10.5 vs. 62.6±11.3, p=0.002, adjusted OR 1.08 [95% CI 1.01–1.16], p=0.035) and higher NLR (11.9±7.4 vs. 5.9±5.0, p=0.002, adjusted OR 1.14 [95% CI 1.01–1.29], p=0.035) were predictors of severe infection at 3 months. NLR ≥4.04 predicted severe infection at 3 months with a sensitivity of 95% and specificity of 52% and the AUROC curve was 0.0794 (95% CI 0.647–0.900). Nine patients died within the first year. Severe infection at 3 months was independently associated with mortality within the first year (OR 6.19 [95% CI 1.12–34.32], p=0.037).
ConclusionsNLR at diagnosis was an independent predictor of severe infection within the first 3 months after immunosuppression start, and severe infection within the first three months was consequently correlated with one-year mortality. NLR is an easily calculated and low-cost laboratory inflammation biomarker and can prove useful in identifying AAV patients at risk of infection and poorer prognosis.
La vasculitis asociada a anticuerpos (VAA) contra citoplasma de neutrófilos es una enfermedad multisistémica. A pesar de la mejora de la tasa de mortalidad desde la introducción de la inmunosupresión, el pronóstico a largo plazo sigue siendo incierto no solo por la actividad de la enfermedad sino también por los efectos adversos asociados al tratamiento. Se ha demostrado que la proporción entre neutrófilos y linfocitos (PNL) es un marcador de inflamación en varios contextos. En este estudio se propuso investigar la capacidad de pronóstico de la PNL en pacientes con VAA.
MétodosSe realizó un análisis retrospectivo de las historias clínicas de todos los pacientes adultos con VAA ingresados en el Departamento de Nefrología y Trasplante Renal del Centro Hospitalario Universitario Lisboa Norte entre enero de 2006 y diciembre de 2019. La PNL se calculó en el momento del ingreso. Los resultados medidos fueron la infección grave a los 3 meses y la mortalidad al año. La capacidad de pronóstico de la PNL se determinó utilizando la curva de características operativas del receptor (COR). Se definió un valor de corte como el de mayor validez. Todas las variables se sometieron a un análisis univariado para determinar los factores estadísticamente significativos que pueden tener resultados. En el análisis multivariado se utilizaron solo las variables que diferían significativamente, empleando el método de regresión logística.
ResultadosSe registraron 45 casos de VAA. La edad media en el momento del diagnóstico era de 67,5±12,1 años y 23 pacientes eran hombres. La media de la puntuación de actividad de la vasculitis de Birmingham (BVAS) en el momento de la presentación era de 26,0±10,4. Veintinueve pacientes dieron positivo en MPO-ANCA, 7 dieron positivo en PR3-ANCA y 9 dieron negativo en vasculitis asociada a ANCA. En el momento del ingreso, el nivel de creatinina sérica (SCr) media era de 4,9±2,5mg/dl, la velocidad de sedimentación globular (VSG) era de 76,9±33,8mm/h, el nivel de hemoglobina era de 9,5±1,7g/dl, el nivel de proteína C reactiva era de 13,2±5,8mg/dl y la PNL era de 8,5±6,8. Treinta y cinco pacientes se trataron con ciclofosfamida y 8 con rituximab para un tratamiento de inducción. Veinte pacientes desarrollaron una infección grave en los primeros 3 meses después de iniciar la inmunosupresión por inducción. En un análisis multivariado, una edad superior (73,6±10,5 frente a 62,6±11,3, p=0,002, OR ajustado 1,08 [IC del 95%: 1,01-1,16], p=0,035) y una PNL superior (11,9±7,4 frente a 5,9±5,0, p=0,002, OR ajustado 1,14 [IC del 95%: 1,01-1,29], p=0,035) fueron indicadores de una infección grave a los 3 meses. Una PNL≥4,04 predijo una infección grave a los 3 meses con una sensibilidad del 95% y una especificidad del 52% y la curva AUROC fue de 0,0794 (IC del 95%: 0,647-0,900). Nueve pacientes fallecieron en el primer año. La infección grave a los 3 meses estaba asociada de manera independiente a la mortalidad en el primer año (OR 6,19 [IC del 95%: 1,12-34,32], p=0,037).
ConclusionesLa PNL en el momento del diagnóstico fue un indicador independiente de la infección grave en los primeros 3 meses después del inicio de la inmunosupresión. La infección grave en los primeros 3 meses se correlacionó como consecuencia con la mortalidad al año. La PNL es un biomarcador de inflamación para pruebas analíticas fácil de calcular y de bajo coste, y puede resultar útil para identificar a los pacientes con VAA que corren riesgo de contraer una infección y tienen un pronóstico más desfavorable.
ANCA-associated vasculitis (AAV) is defined by the 2012 Chapel Hill Consensus Conference Nomenclature of Vasculitides (CHCC 2012) as necrotizing vasculitis, predominantly affecting small vessels, with few or no immune deposits.1 AAV is a systemic inflammatory disease associated with myeloperoxidase (MPO) ANCA or proteinase 3 (PR3) ANCA, although approximately 10% of patients are ANCA negative.2
Clinical features may arise as nonspecific symptoms such as fatigue, anorexia, weight loss, arthralgias, myalgias and fever, with other clinical manifestations depending on the organs affected by vasculitis. Clinical and pathological findings subdivide AAV in three different clinical syndromes: microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA) and eosinophilic granulomatosis with polyangiitis (EGPA).3
Renal involvement is frequent in AAV, particularly in patients with MPA and GPA, and clinical presentation typically includes microscopic hematuria, low grade to sub-nephrotic proteinuria and/or a decline of kidney function frequently presenting as rapidly progressive glomerulonephritis.4,5
Prognosis has evolved favorably since the introduction of corticosteroids first and cyclophosphamide after, with mortality rates dropping from 80% in the first year to approximately 20% in most studies on a five year follow up.6 However, end-stage kidney disease and short-term mortality within one year after diagnosis remain important issues to address, particularly in those who present with rapidly progressive glomerulonephritis and alveolar hemorrhage.7
Predictors of clinical outcomes in AAV have paramount importance in determining which patients may benefit from more aggressive immunosuppressive therapy. Therefore, genetic, histopathological and clinical predictors have been researched, although validation in multicenter randomized controlled trials of these predictors remains scarce.8–10
The neutrophil-lymphocyte ratio (NLR) is a biomarker of systemic inflammation which has shown prognostic ability in multiple clinical settings, namely in progression of chronic kidney disease,11 acute kidney injury,12 cancer patients13 and in renal transplantation.14 Whether the same prognostic ability can be extended to AAV remains unknown.
The aim of this study was to evaluate the prognostic ability of the NLR at admission in patients presenting with de novo AAV for development of severe infection and mortality within the first year.
Materials and methodsStudy designWe conducted a retrospective analysis of the clinical records of all adult patients with AAV admitted to the Nephrology and Renal Transplantation Department of Centro Hospitalar Universitário Lisboa Norte from January 2006 to December 2019.
The study was approved by the Ethical Committee at the Centro Hospitalar Lisboa Norte, EPE, in agreement with institutional guidelines. Informed consent was waived by the Ethical Committee due to the retrospective and non-interventional nature of the study.
Inclusion criteria included: All patients aged 18 or older who were admitted to the Nephrology Ward of the Department of Nephrology and Renal Transplantation of Centro Hospitalar Universitário Lisboa Norte from January 2006 to December 2019 and diagnosed with de novo AAV presenting as rapidly progressive renal failure were eligible for this study.
Exclusion criteria included: CKD patients already on renal replacement therapy.
VariablesAll variables were collected from electronic and hand-written patient clinical records. All scores and formulas were calculated based on raw clinical data.
The analyzed variables included demographic characteristics (age, gender, and ethnicity), laboratory values at admission (serum hemoglobin (Hb), neutrophil count, lymphocyte count, serum creatinine (SCr), erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), ANCA serology), clinical characteristics at presentation (pulmonary hemorrhage, rapidly progressive renal failure), Birmingham Vasculitis Activity Score,15 need of renal replacement therapy, plasmapheresis, induction and maintenance immunosuppression used, SCr at discharge. NLR was calculated at admission as neutrophil count/lymphocyte count.
Renal function recovery at discharge, leucopenia and severe infection at 3 months, and one-year mortality were the outcomes assessed.
Severe infection was defined as life-threatening organ dysfunction caused by a dysregulated host response to infection 16 or any infection requiring hospitalization according to clinician's judgment.
Renal function recovery was defined as improvement of AKI stage.17
Statistical analysisContinuous variables were presented as the mean±standard deviation and categorical variables as the total number and percentage of cases for each category. The normality of the distribution was assessed using the Kolmogorov–Smirnov test. Variables were compared using Student's t-test for normally distributed continuous variables and chi square test for categorical variables.
Firstly, all variables underwent univariate analysis to determine statistically significant factors that may have contributed to leucopenia, severe infection and mortality. Only variables which significantly differed between groups were used in the multivariate analysis using the Cox logistic regression method. Data were expressed as Odds ratios (OR) with 95% confidence intervals (95%CI).
The prognostic ability of the NLR was determined using the receiver operating characteristic (ROC) curve. A cut-off value was defined as that with the highest validity.
No sensitivity analyses were carried out. Multicollinearity was accounted for. Statistical significance was defined at a p-value (p)<0.05. Analyses were performed with the statistical software package SPSS 21.0 for Windows.
ResultsWe registered 45 cases of AAV. The mean age at diagnosis was 67.5±12.1 years, 23 patients (51.1%) were male and the vast majority were Caucasian (97.8%, n=44).
Eighty percent of patients (n=36) presented with rapidly progressive renal failure (RPRF), 25 patients (55.6%) required hemodialysis start, and 13 patients (28.9%) presented alveolar hemorrhage. The mean Birmingham Vasculitis Activity Score (BVAS) at presentation was 26.0±10.4.
At admission, mean serum creatinine (SCr) was 4.92±2.52mg/dL, hemoglobin was 9.57±1.72g/dL, NLR was 8.54±6.83, erythrocyte sedimentation rate (ESR) was 76.90±33.85mm/h and C-reactive protein was 13.2±5.8mg/dL. Twenty-nine patients (64.4%) were ANCA-MPO positive, 7 patients (15.6%) ANCA-PR3 positive and 9 patients (20%) were negative ANCA vasculitis.
Concerning induction therapy, 35 patients (77.8%) were treated with cyclophosphamide, 35 patients (77.8%) with corticosteroid pulses, eight patients (17.8%) with rituximab for induction therapy, of which five were treated with both cyclophosphamide and rituximab. One-third of patients (33.3%, n=15) underwent plasmapheresis.
OutcomesMost patients recovered renal function at discharge (66.7%, n=30), and the majority of patients continued maintenance therapy after induction (64.4%, n=29).
During the first three months after induction immunosuppression start, 10 patients (22.2) developed leucopenia, 20 patients (44.4%) developed severe infection.
Patients who developed severe infection within the first three months of induction immunossupression were older (73.6±10.5 vs. 62.6±11.3, p=0.002), presented more often with alveolar hemorrhage (45 vs. 16%, p=0.033) and higher NLR at admission (11.9±7.4 vs. 5.9±5.0, p=0.002). Fewer patients treated with rituximab as induction therapy developed severe infection (5 vs. 28%, p=0.045). In a multivariate analysis, older age (adjusted OR 1.08 [95% CI 1.01–1.16], p=0.035) and higher NLR (adjusted OR 1.14 [95% CI 1.01–1.29], p=0.035) were predictors of severe infection at 3 months.
On a one-year follow-up, 20% of patients (n=9) died. These patients were older (76.8±7.0 vs. 65.2±12.1, p=0.009; unadjusted OR 1.11 [95% CI 1.02–1.21], p=0.019) and developed more often severe infection within 3 months of induction immunossupression (77.8 vs. 36.1%, p=0.024; unadjusted OR 6.19 [95% CI 1.12–34.32], p=0.037).
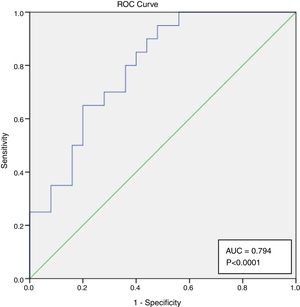
NLR and severe infectionA ROC curve was produced to evaluate the discriminative ability of the NL ratio for severe infection at 3 months after starting induction immunossupression. The AUC for prediction of severe infection at 3 months was of 0.0794 (95% CI 0.647–0.900, p<0.0001) (Fig. 1). The optimal cut-off was assessed to be ≥4.04, which has a sensitivity of 95% and specificity of 52%.
This cut-off was also used to assess disease activity. However, there was no significant association between NLR ≥4.04 and BVAS (p=0.647).
DiscussionIn this retrospective cohort, we demonstrated that a higher NLR at admission was independently associated with development of severe infection within 3 months of starting induction therapy in AAV patients. Additionally, development of severe infection was associated with one-year mortality.
The association of this marker with prognosis in this setting reinforces the role of inflammation in ANCA-vasculitis. Although the events leading to vasculitis initiation remain unproven, several hypothesis have been posed, which highlight the role of genetic, infectious and environmental factors in the pathogenesis of AAV.18
Inflammatory cytokines, such as tumor necrosis factor α and interleukin 1, play a role through priming neutrophils, inducing increased expression of ANCA antigens on their surfaces, enabling ANCAs (formed through prolonged exposure of antigens to the immune system or infection-induced molecular mimicry) to bind them, causing neutrophil activation. Activated neutrophils alter the expression of cell surface adhesion molecules, making possible the attachment to vascular endothelial cells and are responsible for releasing lytic enzymes from granules, reactive oxygen species and neutrophil extracellular traps (NETS), thus provoking damage to the endothelial cells. Moreover, activated neutrophils release factors that activate the alternative complement pathway, generating C5a which amplifies the inflammatory reaction.19–22
NLR has been described in recent literature as a significant marker of inflammation and a useful predictor of the development of acute kidney injury in different settings, namely in the emergency department, sepsis-associated AKI,23,24 contrast-induced AKI,25 after cardiovascular surgery 26 and major abdominal surgery.27
Neutrophil counts are usually proportionally elevated to inflammation, and activated neutrophils are associated with the pathogenesis of AAV.22 Also lymphocyte counts may be diminished in inflammatory states, namely in active AAV in which lymphopenia results from extensive recruitment of peripheral T cells to the affected tissues.28,29 Thus, the NLR could hypothetically reflect the inflammatory load of AAV patients.
Furthermore, neutrophilia and lymphopenia can be a result of immunosuppression or a result of systemic infection developed during immunosuppressive treatment.30 Therefore, to reduce bias the ratio should be calculated at disease presentation before any therapeutic measure takes place.
Validation of this practical, inexpensive and easily calculated biomarker has been explored in AAV patients, with early studies suggesting a positive relation between the NLR and BVAS, acute phase reactants and worse renal outcomes.31 Other studies, however, have shown conflicting results between the ratio and disease activity as well as extrarenal manifestations.32
Recently, Ahn et al. retrospectively analyzed 160 AAV patients and reported that an elevated NLR was correlated with vasculitis activity estimated by BVAS at diagnosis and predicted relapse during follow-up (Patients having NLR ≥5.9 exhibited severe AAV more frequently than those having NLR<5.9 at diagnosis (relative 2.189, p=0.023).).33 In our study, we have not confirmed this association. Park et al. also proposed a platelet to lymphocyte ratio (PLR) to predict AAV inflammatory burden, in which a PLR ≥272.0 was able to predict severe AAV at diagnosis.34
The assessment of clinical predictors of complications associated with the immunosuppressive treatment of AAV has been barely done. On a literature review, there have been no reports of NLR used as a predictor of severe infections in AAV patients. Our study therefore reflects the importance of the NLR as an indirect and sensitive predictor of inflammation and outcomes in this setting.
Although novel therapeutic options have been developed for patients with AAV,35,36 long-term safety still composes a major concern, specifically in terms of infectious complications and neoplasms, since these therapeutics do not appear to significantly decrease the risk of developing such adverse events.37
In fact, infections were responsible for hospitalization of more than 30% of patients treated with immunosuppressive therapy on a five-year follow up and the leading cause of death in those patients.7,38
Age over 65 years, pulmonary involvement and the Five Factor Score (FFS) constitute baseline independent predictors of severe infection according to a retrospective analysis of five major randomized controlled trials (RCTs) of the French Vasculitis Study Group.39 A higher BVAS at diagnosis has also shown prognostic ability in assessing development of serious infection in AAV patients.40 Smoking status and baseline serum creatinine at diagnosis may also play a role as risk factors for infectious complications.41
Data regarding infectious complications related to treatment modalities and strategies remain controversial. Although no therapeutic regimen was significantly associated with severe infection, patients treated with corticosteroids and cyclophosphamide as induction therapy tended to have had more severe infections according to the French Vasculitis Study Group.39,42 The role of rituximab has also been extensively studied in recent years, with two major RCTs showing that serious infections rates were similar between rituximab and the groups including patients treated with iv cyclophosphamide.35,36 Furthermore, concerns regarding corticosteroids adverse effects have led to the development of steroid-sparing protocols with good results, namely reduced risk of infectious complications and without an increase in progression to end-stage renal disease or mortality.43,44
Therefore, corticosteroids sparing regimens, rituximab over cyclophosphamide or mixed protocols where cyclophosphamide is used in reduced cumulative doses are currently used and being developed to achieve a mainstay in therapeutic options, providing the same efficacy achieved with previous regimens but at a reduced adverse events rate. In our cohort, no differences were found between treatment regimens and outcomes, nevertheless the small sample may have resulted in these results.
Mortality also remains an important issue in patients with AAV. Despite important improvements after initiation of corticosteroids and cytotoxic therapy as noted above, an important portion of patients with AAV still decease as a consequence of disease activity or more frequently as consequence of severe infection in the first year.45 On the long term, cardiovascular disease, malignancy and mortality account for the major causes of death in this population.7
Age, secondary infection, initial renal function and pulmonary involvement of AAV act as independent predictors of mortality, as reported in a retrospective study with almost 400 patients.46 Higher BVAS, lower hemoglobin and higher white cell count at presentation were also described as negative outcome predictors in AAV patients.7
Since early mortality plays an important role in this pathology, identifying predictors of premature mortality may help in stratifying those who may be in need for intensive care unit (ICU) admission and more tailored therapy.47 Acute kidney injury, smoking, hemoptysis and respiratory failure were all predictors of ICU admission, with those in need for blood transfusions and mechanical ventilation being at increased risk of early mortality, within 28 days from admission.48 Although multiple clinical disease activity parameters significantly correlate with one-year mortality, a high FFS and the occurrence of severe infection during the first month correlate better and constitute the most solid predictors of one-year mortality.49
In our study, the NLR was a strong predictor of infection. Indeed, a NLR ≥4.04 is associated with a significant risk of severe infection at 3 months. More importantly, assessment of this score emphasizes the low risk of developing severe infection in patients who have NLR <4.04, which could guide therapy decision. Nevertheless, infection was not associated with mortality in the multivariate analysis, which could be a result of the reduced number of patients in our population. Therefore, further studies are required to corroborate the NLR as a predictor of severe infection and mortality in AAV patients.
We address some of the important limitations of this study. Firstly, the single-center nature of our study limits generalizability. Secondly, the retrospective design and the small cohort of patients could have contributed to disregarding some potential confounders with prognostic importance. Thirdly, the NLR was not age-adjusted, which might have overestimated the importance of the ratio in older patients, who are prone to exhibit a pro-inflammatory state. Finally, we did not discriminate infection source and did not address cause-specific mortality as well.
Despite these limitations, our study has several notable strengths. To the best of our knowledge, this is the one of the first studies evaluating the association between the NLR and prognosis in AAV patients. We demonstrated the clinical significance of the NLR at diagnosis in estimating the prognosis of AAV patients. Indeed, the high specificity of the NLR reflects that a high ratio is predictive of severe infection within 3 months. Also, we provided an optimal cutoff of NLR to predict severe infection.
We highlight the need for further understanding of AAV in order to identify at-risk patients, guide immunossupression and ultimately improve patient outcomes.
ConclusionIn conclusion, we demonstrated that the NLR at admission was independently associated with development of severe infection within 3 months of starting induction therapy in AAV patients. This ratio can be quickly assessed from a routine blood sample and can prove useful in identifying patients at risk of infection. Recognizing high risk patients is crucial to guide immunosuppression and improve patient outcomes.
Ethics approval and consent to participateThe study was approved by the Ethical Committee at the Centro Hospitalar Universitário Lisboa Norte, EPE, in agreement with institutional guidelines. Informed consent was waived by the Ethical Committee due to the retrospective and non-interventional nature of the study.
Consent for publicationNot applicable.
Availability of data and materialPlease contact author for data requests.
FundingNo funding was received for this study.
Authors’ contributionsJAF and JG made substantial contributions to the study concept and design, analysis and interpretation of data, and were involved in drafting the manuscript and revising it critically for important intellectual content. JAF, ID, JG participated in the acquisition of data. SJ and JAL were involved in the critical revision of the manuscript and approval of the final version to be submitted.
Conflict of interestsThere is no conflict of interest. The results presented in this paper have not been published previously in whole or part, except in abstract format.
None.