Anticoagulant use is essential to safe hemodialysis (HD) therapy because it prevents thrombosis in the extracorporeal circuit. For HD therapy in Japan, nafamostat mesilate (NM) is usually used during HD in patients with a tendency to bleed because of its short half-life.1 However, NM use is reportedly associated with adverse effects, including hyperkalemia,2,3 agranulocytosis,4 and anaphylactic reaction.5,6 A recent report indicated the development of anaphylactic shock in HD patients who received NM.6 Here, we report a case of NM-associated anaphylactic reaction in a patient on HD, followed by severe intestinal edema.
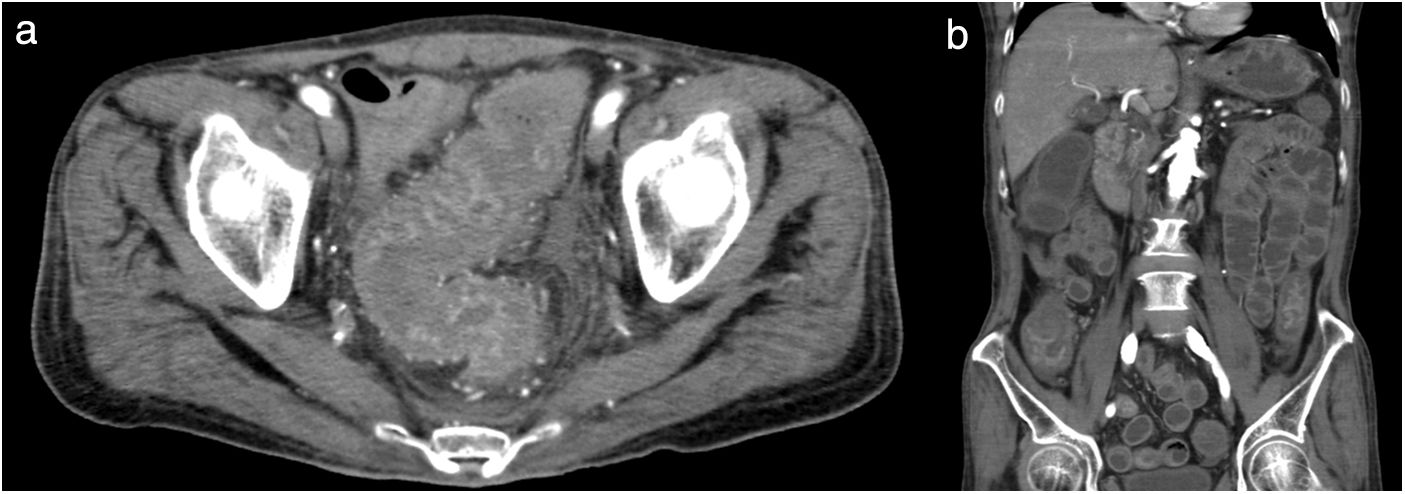
A 64-year-old man with diabetes mellitus and a 5-year history of HD was admitted to our hospital for body-fluid status evaluation because of a recent episode of intradialytic hypotension at his dialysis facility. The intradialytic hypotension was suspected to be associated with ultrafiltration-induced hypovolemia during HD before admission. NM was used several times instead of heparin sodium for retinal bleeding complicated by diabetes mellitus in his dialysis facility. At our dialysis center, HD was performed in the same manner using NM. We carefully monitored for the occurrence of ultrafiltration-induced intradialytic hypotension. His blood pressure (BP) was 195/90mmHg before HD but rapidly decreased to 114/56mmHg. The arterial oxygen saturation measured by pulse oximetry (SpO2) was 88% with dyspnea and deterioration of consciousness shortly after the HD initiation. Based on the clinical course from before admission to this episode, intradialytic hypotension with NM-associated anaphylactic reaction was diagnosed. At a later date, specific immunoglobulin E (IgE) antibodies to NM were detected in his blood. The HD therapy was discontinued to avoid further worsening of his systemic circulation. Although his BP recovered and his symptoms disappeared after HD was discontinued, he suffered from mild abdominal tenderness after the anaphylactic reaction; therefore, contrast computed tomography of the abdomen and pelvis was performed, which revealed remarkable mucosal edema at a circumference of the sigmoid colon (Fig. 1a) and along the entire intestine on a coronal view (Fig. 1b). During the next HD session using low-molecular-weight heparin, the intradialytic hypotension completely disappeared; thereafter, HD was safely performed. However, because the intestinal edema persisted on lower gastrointestinal endoscopy 2 weeks after the reaction, total parenteral nutrition instead of solid and liquid foods was necessary for approximately 3 weeks. Thereafter, he ate normally without worsening of the intestinal edema and fully recovered after 1 month of treatment.
An anaphylactic reaction usually occurs by an IgE-dependent immunologic mechanism and is commonly triggered by foods, stinging insect venom, and medications through acute onset within minutes to several hours. Furthermore, its reaction frequently increases vascular permeability, which increases the risk of death due to fluid extravasation with resultant sequential decreases in circulating blood volume, venous return, and BP.7 Interestingly, the increase in vascular permeability induced by the anaphylactic reaction differs among systemic organs, and the extent of vascular permeability changes vary considerably among tissues: vascular permeability increases markedly in the trachea, moderately in the mesentery and intestines, and slightly in the other organs.8 In this case, the whole-intestine edema associated with increased vascular permeability was surprisingly remarkable compared to other systemic organs in the NM-associated anaphylactic reaction, although the presence of tracheal edema might be suspected because of his respiratory distress and the SpO2 decrease at the reaction's onset. In addition to the intestinal edema, it was interesting that improvement of the intestinal edema and dysfunction took several weeks. The reason for the difference between the rapid improvement of the circulating status and respiratory distress and the sustained gastrointestinal symptoms, including intestinal edema, in this case remains unclear. However, reports to date of the association between NM-associated anaphylactic reactions and remarkable intestinal edema are scarce. Therefore, this case report is relevant and showed that we should carefully monitored the systemic vascular permeability increase caused by the anaphylactic reaction, particularly in the intestine, in patients in whom NM is used during HD.
In Japan, the use of NM as an anticoagulant during blood purification is recommended in patients with various hemorrhagic complications as it has a lower risk of hemorrhage.1 However, in patients with a history of NM-induced allergic reactions, low-molecular-weight heparin is usually administered instead of NM.5 In this case, HD was performed without worsening of the retinal bleeding after switching to low-molecular-weight heparin. Furthermore, rinsing the dialysis circuit with heparin and albumin9 and introducing a regional citrate infusion into the circuit10 have recently shown good survival of the extra corporeal circuit without increasing the bleeding risk. Therefore, HD may have been better performed using these methods to avoid the occurrence of the NM-associated anaphylactic reaction.
In conclusion, our experience described here suggests that patients administered NM during HD because of hemorrhagic complications should be carefully observed for anaphylactic reaction including BP reduction, respiratory distress, and intestinal edema via increased vascular permeability.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank our hospital's dialysis staff members.