Pulmonary-renal syndrome (PRS) is defined as pulmonary and renal failure, and is caused by immunological and non-immunological diseases. Although the most frequent immunological causes for PRS are small vessel vasculitis and lupus,1 other causes have to be considered.
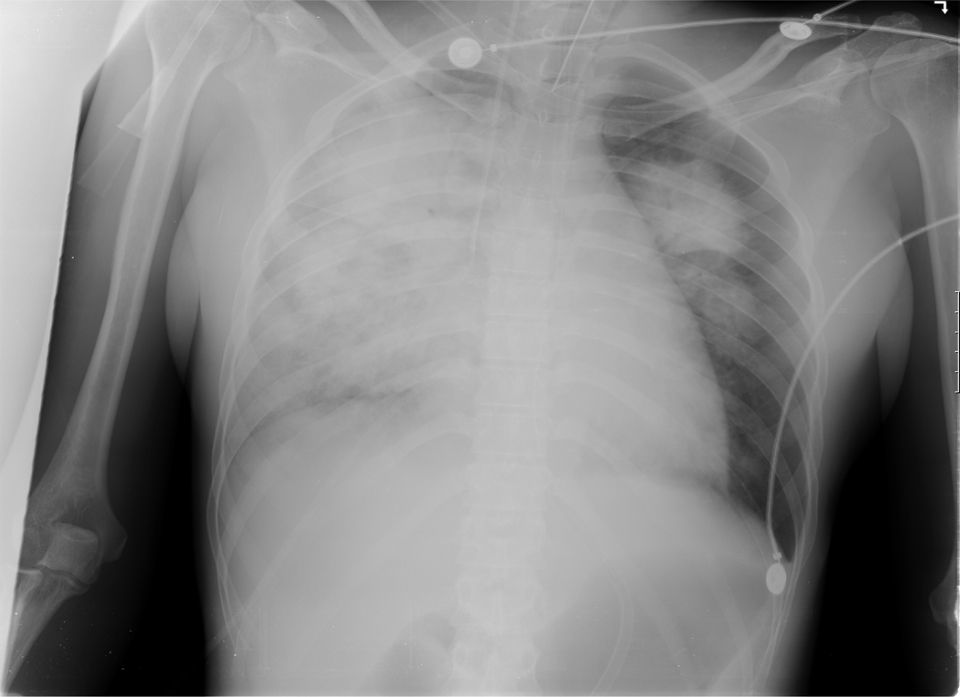
Here, we present the case of a 35-year-old, black, previously healthy woman referred from Cape Verde to our Department to investigate renal failure (creatinemia 2.5mg/dL), hypertension, lower limbs edema, foamy urine, anemia (Hb 11.5mg/dL), and bilateral arthralgias developing within the last 6 months. She had no family history of renal disease neither respiratory, neurologic or gastrointestinal symptoms nor alopecia, rash, oral ulcers, photosensitivity, hematuria or recent drug use. At admission, she was polypneic, hypertensive, aeodematous, oligoanuric, presented bilateral fine pulmonary crackles, and bilateral erythematous lesions in thighs. Laboratory revealed anemia (Hb 7.1g/dL), leukocytosis with neutrophilia (16.670/mm3; 91.9%), elevated C-reactive protein (12.2mg/dL), elevated erythrocyte sedimentation rate (70mm 1st h), renal insufficiency (uremia 247mg/dL, creatininemia 7.5mg/dL), and of NT pro-BNP (>105000pg/mL). Blood gas test showed severe hypoxemia. Urinalysis showed haematuria (200/mm3) and proteinuria 300mg/dL, without casts. Chest X-ray revealed bilateral diffuse opacities (Fig. 1), and chest CT suggested pulmonary hemorrhage. Ultrasonogram showed normal sized kidneys with increased echogenicity. She required mechanical ventilation and hemodialysis. Bronchofibroscopy revealed alveolar hemorrhage, and plasmapheresis was initiated.
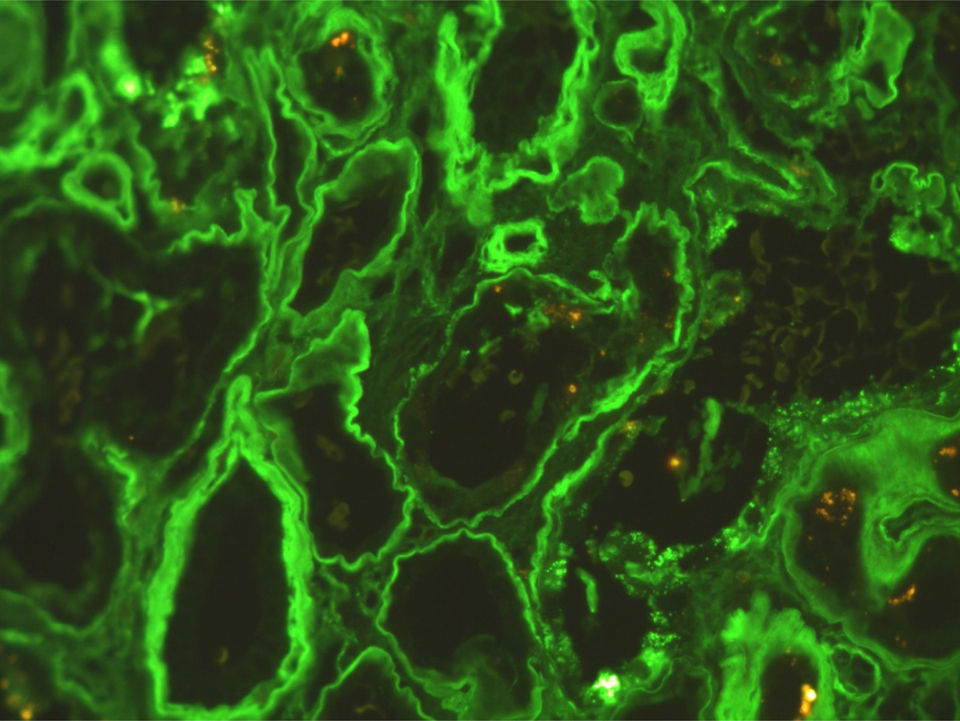
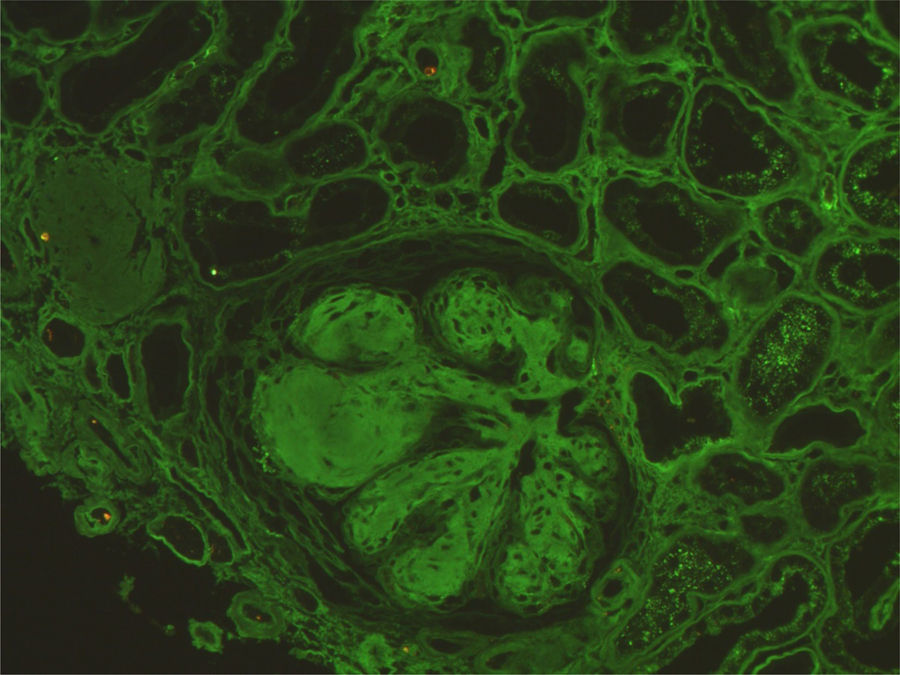
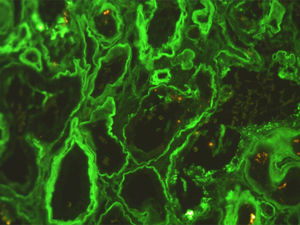
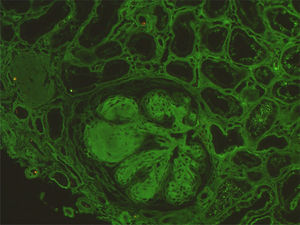
Complementary investigation showed nephrotic range proteinuria (4.7g/24h). Serum protein electrophoresis showed an alpha2 spike and hypogamaglobulinemia. Serum complement was normal and serology for lupus, vasculitis and cryoglobulinemia, as well as for human immunodeficiency virus, hepatitis B and C infections were negative. Echocardiogram revealed a type II diastolic dysfunction. A renal biopsy was performed and revealed nodular glomerulosclerosis. Immunofluorescence revealed linear staining for kappa light chains along the tubular basement membrane and also in the glomerulus, allowing the diagnosis of light chain deposits disease (LCDD) (Figs. 2 and 3). Serum immunofixation revealed a kappa light chain band, and urine immunofixation revealed Bence-Jones kappa. Bone marrow biopsy and aspirate showed normocellular marrow with 10% monoclonal plasmocytosis. Therefore, multiple myeloma was diagnosed. She received chemotherapy and an autologous hematopoietic cell transplant, achieving maintained complete hematological response. At one-year of follow-up, she remains dialysis-dependent.
The initial presentation led us to consider an immunological cause for the pulmonary renal syndrome, despite the negative immunological results, which might occur in 10–20% of the PRS of immunological origin. The unexpected nodular glomerulosclerosis on the kidney biopsy led us to further investigate an hematological disease, because together with diabetes mellitus and smoking, light or heavy chain deposits disease is one of the main causes of nodular glomerulosclerosis. The investigation of the hematological disease was consistent with a diagnosis of multiple myeloma, according to the International Myeloma Working Group criteria.2
LCDD is a rare renal manifestation of plasma cell disorders.3 LCDD is a systemic disease with renal, cardiac, pulmonary, hepatic and gastrointestinal involvement.3–5 Renal involvement is the most frequent and manifests as nephrotic syndrome and renal insufficiency, typically rapidly progressive. Renal biopsy typically reveals nodular glomerulosclerosis and thickening of the tubular basement membrane. In 80% of the cases it is characterized by the deposition of kappa light chains along the glomerular capillaries, nodules and the tubular basement membrane. Electron microscopy reveals granular deposits.4,6
Although we cannot conclude on the cause of the pulmonary hemorrhage, because of the lack of pulmonary biopsy, we speculate on pulmonary tissue LC deposition, as this seems to be a case of systemic LC deposition: kidney, lung, heart, skin and articulations. This case highlights that LCDD should be kept in mind in the differential diagnosis of PRS.
The authors would like to acknowledge Helena Viana, MD and Fernanda Carvalho, MD, who were responsible for the kidney biopsy results and supplied the images.