Antecedentes: Recientemente, se ha sugerido que la nicotinamida es como un fármaco eficaz para la hiperfosfatemia en pacientes en hemodiálisis. Los autores evaluaron la eficacia y la seguridad de la nicotinamida en estos pacientes con dosis más bajas y mayor duración que en otros estudios. Métodos: Cuarenta y ocho pacientes con fósforo sérico en ayunas >5 mg/dl participaron en este estudio clínico aleatorio y fueron asignados al azar a dos grupos de igual tamaño uno de los cuales recibiría nicotinamida y el otro, placebo. El estudio duró 8 semanas. En las primeras 4 semanas, se les administraron 500 mg de nicotinamida al día, y en el segundo período de 4 semanas se aumentó la dosis a 1.000 mg/día. Se tomaron muestras de sangre en la primera, quinta y novena semanas. Resultados: En el grupo de la nicotinamida, el nivel de fósforo se redujo de 5,9 ± 0,58 a 4,77 ± 1,43 mg/dl en la quinta semana (p = 0,002) y a 4,66 ± 1,06 mg/dl en la novena semana (p = 0.000). El producto calcio-fósforo se redujo significativamente siguiendo el mismo patrón que el fósforo. El nivel de HDL aumentó de 42,46 ± 8,01 a 55,71 ± 11,88 mg/dl en la quinta semana (p = 0,000) y a 65,25 ± 20,18 mg/dl en la novena semana (p = 0,000). Los niveles de calcio sérico, ácido úrico, TGO, TGP e iPTH no cambian de manera significativa. En comparación con la primera semana, el recuento de plaquetas en la quinta y novena semana disminuyó. No se observaron cambios significativos en el grupo placebo. Conclusiones: En nuestros pacientes, la nicotinamida disminuyó de forma efectiva el fósforo, aumentó el HDL, y causó trombocitopenia. Como la nicotinamida redujo el recuento de plaquetas y causó trombocitopenia en dosis más bajas que en otros estudios con estos pacientes, es necesario planificar más estudios para evaluar la seguridad del fármaco, especialmente en diferentes poblaciones.
Background: Recently, nicotinamide has been suggested as an effective drug for hyperphosphatemia in hemodialysis patients. The authors assessed the efficacy and safety of nicotinamide in these patients with lower doses and longer duration than other studies. Methods: Forty eight patients with fasting serum phosphorus >5 mg/dl enrolled in this randomized clinical trial study and were randomly assigned to two equal-sized groups of nicotinamide or placebo. The study lasted 8 weeks. In the first four weeks, nicotinamide was administered at 500 mg/day, and in the second four weeks at 1,000 mg/day. Blood samples were tested at baseline, week 4, and week 8. Results: In nicotinamide group, the mean phosphorus level decreased from 5.9 ± 0.58 mg/dl to 4.77 ± 1.43 mg/dl in week 4 (P = 0.002) and to 4.66 ± 1.06 mg/dl in week 8 (P = 0.000). The mean calcium-phosphorus product decreased significantly with the same pattern as phosphorus. High-density lipoprotein level increased from 42.46 ± 8.01 mg/dl to 55.71 ± 11.88 mg/dl in week 4 (P = 0.000) and to 65.25 ± 20.18 mg/dl in week 8 (P = 0.000). Levels of serum calcium, uric acid, SGOT, SGPT, and iPTH didn’t change significantly. Compared to baseline, the platelet counts were decreased in both week 4 and week 8. No significant changes were observed in placebo group. Conclusions: In our patients, nicotinamide effectively decreased phosphorus, increased high-density lipoprotein, and caused thrombocytopenia. Since nicotinamide lowered platelet counts and caused thrombocytopenia in lower doses than other studies in these patients, it is necessary to plan other studies for assessing the safety of the drug especially in different populations.
INTRODUCTION
Hyperphosphatemia is a significant problem in hemodialysis patients; in fact, renal failure is the main cause of it. Control of serum phosphorus in hemodialysis patients is very important because hyperphosphatemia and the increase in calcium-phosphorus product are associated with overall and cardiovascular mortality in these patients1,3. There are different measures to control hyperphosphatemia such as restriction in dietary phosphorus, dialysis, and using calcium or non-calcium phosphate binders. But none of these can control hyperphosphatemia as recommended4-7. Physicians have been using nicotinamide (= niacinamide) for different diseases for many years. Nicotinamide inhibits sodium-phosphorus co-transporting (Na/Pi2) in both renal proximal tubules and intestine8,9; as a result, the absorption of phosphorus by related cells is reduced. Recently, nicotinamide has been reported to be effective and safe in controlling hyperphosphatemia in hemodialysis patients, either alone or as an additive therapy10,11.
Several studies have been carried out to evaluate the effects of nicotinamide or nicotinic acid in reducing phosphorus in hemodialysis patients10-14. All of these have demonstrated that they are effective. It has been shown that nicotinamide is also effective in increasing high-density lipoprotein (HDL) and decreasing low-density lipoprotein (LDL) in some studies10,11. However, gastrointestinal symptoms or changes in platelet counts have been considerable issues with nicotinamide administration10,11,15. In Iran, the main procedure for controlling hyperphosphatemia is administering calcium phosphate binders. But because of hypercalcemia as an adverse effect6, they can’t be administered in large doses. Therefore, it is administered in low doses bearing no desired effects. On the other hand, the alternative use of non-calcium phosphate binders such as Sevelamer or Lanthanum is cost-inhibitive for most patients. Nicotinamide is both cheaper and more effective in lower doses than most phosphate binders. With this knowledge, an 8-wk double-blind, placebo-controlled, parallel randomized clinical trial was designed to evaluate the effects and safety of nicotinamide in lower doses and longer duration (500 mg/day for the first four weeks and 1,000 mg/day for the second four weeks of the study) in hemodialysis patients.
MATERIAL AND METHODS
Subjects and randomization
The study protocol was approved by the ethics committee of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. The study was performed at two hemodialysis centers in Ahvaz. The inclusion criteria were: Age ≥18 years, fasting serum phosphorus ≥5 mg/dl, maintaining on hemodialysis for more than 2 months, and constant dosage of phosphate binders during past 2 weeks. The exclusion criteria were: history of hepatic disease or significant disturbance in liver tests, active peptic ulcer, recent treatment with niacin or nicotinamide, non-cooperation, malignancy, and absence from hemodialysis sessions.
The study was explained to the subjects and written informed consents were obtained.
They were randomly assigned to two groups, placebo group (n = 24) and nicotinamide group (n = 24). The nicotinamide group was the treatment group of the study in which the nicotinamide tablets were used as well as their usual doses of calcium carbonate. Placebo group was the control group of the study and these patients took placebo as well as their usual doses of calcium carbonate (the calcium carbonate dose must have been stable during the past 2 weeks before beginning the study and was kept constant during the study in all subjects unless it was necessary for the research committee to decide to change the dose in any of them according to the study protocol). Placebo and immediate release nicotinamide tablets (500 mg) were prepared by consultant pharmaceutist of the study at the industrial laboratory of pharmacy faculty of the university using nicotinamide powder purchased from Saveh Novin Kavosh Company. All required quantitative and qualitative control tests were performed according to US Pharmacopoeia.
Study design
This was a double-blind, placebo controlled, parallel clinical trial. The subjects were recruited at two dialysis center in Ahvaz. Reviewing previous studies for changes in phosphorus revealed that the mean difference and the standard deviation (SD) were nearly equal, and at 0.05 significance level and %90 power, the required sample size was calculated to be 44 (22 in each group).
After assessing inclusion and exclusion criteria, 48 patients who were eligible enrolled in the study and randomly assigned to two equal-size groups. The study lasted 8 weeks from late January till March 2010; during the first four weeks, nicotinamide was administered 500 mg/day, and then it was administered 1,000 mg/day in weeks 5 to 8. In case of adverse effects, phosphorus level ≤3.5 or >8 mg/dl, thrombocytopenia (platelet counts less than 150,000/mm3), or clinical findings of low platelet count, the research committee decided on adjusting the dose or other necessary measures.
Fasting serum samples were drawn before the first dialysis session of the week for biochemistry and lipid profile tests at baseline, week 4, and week 8. Also, blood samples were drawn for complete blood counts at the same time. Tests were performed for phosphorus, calcium, uric acid, HDL, LDL, triglyceride, cholesterol, SGOT, SGPT, iPTH, and platelet counts. All tests were done using standard laboratory instruments and kits.
The primary end point was the change in serum phosphorus in week 4 and week 8, and secondary end points were the changes in other studied variables.
Results are expressed as mean ± SD. For qualitative variables such as sex, Chi-square or Fisher’s exact test, and for quantitative variables, repeated measures analysis of variance (both within-subjects for finding the significance of changes within each group and between-subjects for finding the overall difference between the two groups during the study) was performed. Since iPTH was measured just two times (at the beginning and at the end of the study), t-test (comparing differences between two groups) and paired t-test (comparing differences within each group) were used. For t-test, in rare instances, where Levene’s test was significant, unequal variances t-test was used. P-values less than 0.05 were considered statistically significant.
RESULTS
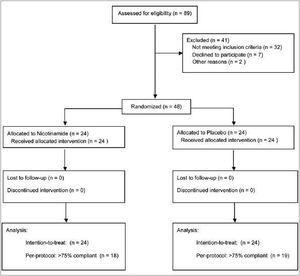
Forty-eight eligible subjects participated and all completed the study. Thirty-seven of them were ≥75% compliant according to pill counts. Patients flow is shown in figure 1.
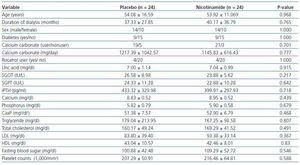
Twenty-two (91.6%) of the subjects in nicotinamide group and 21 (87.5%) in placebo group had three sessions of dialysis per week (the others had two sessions per week). The duration of dialysis in any session was 4 hours. Patients had residual renal function of <10 cc/min. Dialysate concentration in calcium was similar for all patients (In these centers, only one kind of dialysate is used). The types of vascular access were via arteriovenous fistula or arteriovenous graft. Demographic and baseline data are shown in table 1.
Table 1 reveals that at baseline the two groups are similar according to the mean values of different variables.
Drug efficacy
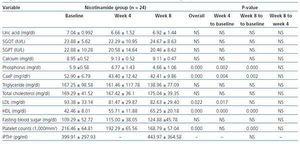
In table 2 the changes in variables levels are displayed for nicotinamide group. Using within-subjects repeated measures analysis of variance, the three stages of measurements (baseline, week 4, and week 8) are compared. The overall changes of phosphorus (p = 0.000), calcium-phosphorus product (p = 0.000), HDL (p = 0.000), LDL (p = 0.022), and platelet counts (p = 0.000) were statistically significant for three measurements in nicotinamide group. Pairwise comparisons using Bonferroni adjustment in repeated measures analysis of variance are also shown in the related columns in the table. Bonferroni adjusted comparisons of week 4 to week 8 were not significant for any of the variables. It was not significant for comparing baseline and week 4 for platelet counts (p = 0.065), too.
No statistically significant overall changes were found for any of the variables in within-subjects repeated measures analysis of variance for placebo group. The pairwise comparisons were statistically non-significant, too.
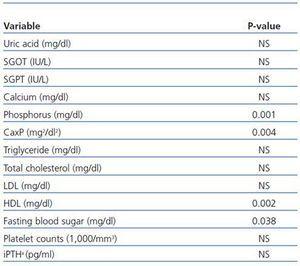
Between-subjects repeated measures analysis of variance was applied to compare the overall changes in nicotinamide group in three stages of measurements with those of placebo group. The results are shown in table 3. They are statistically significant for comparing the overall changes between the two groups for phosphorus (p = 0.001), calcium-phosphorus product (p = 0.004), HDL (p = 0.002), and fasting blood sugar (p = 0.038). For platelet counts, although the changes in nicotinamide group were statistically significant and clinically important, the overall differences between the two groups were not significant. This may be due to slight reduction in platelet counts in placebo group during the study.
Figure 2 shows the changes for important variables in two groups graphically.
Twenty days after the end of the study, another platelet counts test was performed for nicotinamide group. The mean platelet counts had increased considerably at this stage, and the difference between mean platelet counts at this stage and week 8 was significant (p = 0.027). It had no statistical difference from baseline platelet counts (p = 0.503).
Adverse effects (drug safety)
Although eight weeks of study is not long enough for safety reports, the following adverse effects were observed during the study.
Thrombocytopenia
Two subjects in nicotinamide group had thrombocytopenia in week 4 which were in the normal range in the next checking laboratory tests. In week 8, six patients (25%) in nicotinamide group had thrombocytopenia, two of whom had less than 100,000/mm3 and retardation in hemostasis at the site of dialysis needles in week 8 which were relieved after discontinuation of the drug. Two other patients in this group had 150,000/mm3 platelet counts, less than their initial counts. None of the placebo group subjects had thrombocytopenia in week 4 or 8.
Diarrhea
It occurred in 4 subjects (16.6%) in nicotinamide group. One happened at the end of week 4, with titrating from 500 to 1,000 mg/day, and three others occurred near the end of the study; however, the condition was resolved by lowering the dose.
Hyperglycemia
The changes in fasting blood sugar levels are noticeable in nicotinamide group. Although, the changes are not statistically significant, they are clinically important. In placebo group, the changes in fasting blood sugar are not considerable. The differences between the two groups are statistically significant.
Flushing
No cases of flushing occurred.
For two of the patients in placebo and one in nicotinamide group who had serum phosphorus levels more than 8 at the end of week 4, the research committee decided to increase the dose of calcium carbonate by 500 mg/day. After that, checking serum phosphorus showed acceptable values. In nicotinamide group, the dose of nicotinamide was lowered for five patients because of induced diarrhea (four patients, explained above) or fasting serum phosphorus level less than the safety point (one patient).
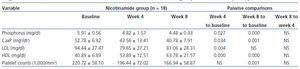
All above mentioned analyses were performed for 37 subjects who were >%75 compliant during the study. In nicotinamide group, the overall comparisons of three stages of measurements using within-subjects repeated measures analysis of variance showed statistically significant differences for phosphorus (p = 0.001), calcium-phosphorus product (p = 0.002), HDL (p = 0.000), LDL (p = 0.016), and platelet counts (p = 0.000). Pairwise comparisons are shown in table 4 for variables with statistically significant changes. The changes for other variables in nicotinamide group were not statistically significant and had the same increasing or decreasing patterns as those of intention-to-treat analysis. No significant changes were found in placebo group. Between-subjects repeated measures analysis of variance showed statistical significant differences between the two groups for phosphorus, calcium-phosphorus product, and HDL. For the sake of brevity, the results are not shown.
DISCUSSION
Nicotinamide, a form of vitamin B3, is a component of NAD (Nicotinamide Adenine Dinucleotide) and NADP (Nicotinamide Adenine Dinucleotide Phosphate) which are important co-enzymes for enzymatic oxidation-reduction reactions pathways16. High doses (>3 g/day) of nicotinamide may cause hepatotoxicity. No significant changes were found in liver enzymes levels during this study, possibly due to lower doses and shorter duration of administration.
Nicotinamide reduces the expression of Na/Pi2 transporter at intestinal brush border17 and thereby inhibits sodium-phosphate co-transporting18. Some non-controlled studies showed that nicotinamide or nicotinic acid can reduce phosphorus levels in hemodialysis patients10,12-14. Recently, Cheng et al., in a placebo-controlled clinical trial study considered the effects and safety of nicotinamide as addditive with other phosphate binders in these patients11. These studies declare that nicotinamide can reduce serum phosphorus in hemodialysis patients either when used alone or as additive. In the present study, nicotinamide significantly reduced serum phosphorus. Most changes happened with 500 mg/day for four weeks (p = 0.000). With 1,000 mg/day nicotinamide during the second four weeks, there were no considerable changes. The question still remains if 500 mg/day continued for the second four weeks, would the results be the same or nearly the same as those of 1,000 mg/day?
The authors found significant results with doses lower than those of others. Of course, some have reported the most changes (but not significant) to be with lower doses11. The authors assume, at least in our population, that lower doses of nicotinamide are effective in lowering serum phosphorus in hemodialysis patients if administered for long-term duration. Perhaps differences among populations (racial, geographical, nutrition patterns and other factors) are the causes.
Calcium-phosphorus product decreased significantly during the study. The changes were in accordance with phosphorus changes.
Fasting blood sugar changes were not significant but they were considerable in nicotinamide group. Other studies haven’t investigated the effects of nicotinamide on blood glucose. But niacin, especially in high doses, increases blood glucose mainly via increasing insulin resistance19-21. It needs further investigations.
Uric acid levels have not changed significantly during this study. Most previous studies have not mentioned significant or considerable changes in levels of uric acid, too11-14.
In some articles, significant or considerable changes in HDL and LDL have been reported10,11. Actually, niacin decreases plasma fatty acids, triglyceride, and LDL by reducing lipase effects in adipose tissue and presumably increases HDL concentration by increasing apoA-1 which is the main lipoprotein of HDL22. It has been shown that nicotinamide, with higher doses than niacin, can decrease triglyceride, cholesterol, and fatty acids in rats23. Takahashi et al. showed that nicotinamide can increase HDL and decrease LDL in hemodialysis patients10. A non-significant increase in HDL, but no change in LDL and triglyceride has been found in a recent randomized clinical trial11. The present study shows that nicotinamide can increase HDL (statistically significant even with a low dose of 500 mg/day for 4 weeks), and decrease the values of triglyceride and LDL with clinical significance. No noticeable changes were found in cholesterol levels.
Although eight weeks of study is not long enough for safety reports, some adverse effects were observed during the study which will be explained below.
In this study, decrease in platelet counts is statistically significant in nicotinamide group. Although the overall differences for platelet counts were non-significant between the two groups, this does not reject the importance of such a clinical finding in nicotinamide group. In nicotinamide group, thrombocytopenia occurred in 6 (25%) patients after 8 weeks of study; two of which were lower than 100,000/mm3. No thrombocytopenia occurred in placebo group. In some other studies, although with higher doses and longer duration, thrombocytopenia has been a major concern with nicotinamide11,15. Twenty days after the end of the study, the mean platelet counts had significantly increased (from 168,000/mm3 to 200,000/mm3) in nicotinamide group. The mechanism for decreasing platelet counts by nicotinamide is unclear, but for niacin, it may be due to decrease of TBG.
Diarrhea is another side effect of nicotinamide10,11. Diarrhea was present in 4 (16.6%) of the subjects in nicotinamide group which were all relieved after lowering the dose. Therefore, diarrhea like thrombocytopenia might be dose-related in this study. No cases of diarrhea happened in placebo group.
CONCLUSION
The authors conclude that nicotinamide is an effective drug to reduce phosphorus and calcium-phosphorus product, and to increase HDL in these patients significantly. It also reduces LDL and triglyceride, and increases fasting blood sugar with clinical importance. Administering a drug like nicotinamide for lowering serum phosphorus, as additive or as an alternative, is reasonable in our hemodialysis patients who have to take calcium phosphate binders with their known side effects.
In this study, nicotinamide could decrease platelet counts significantly. Decrease in platelet counts and thrombocytopenia with nicotinamide are very important issues that must always be a concern in administering it. In fact, the authors believe that the safety of nicotinamide (especially reduction in platelet counts and thrombocytopenia) needs to be assessed more.
Nicotinamide was effective in lowering mean fasting serum phosphorus down to an acceptable level in 500 mg/day dose. The authors assume that the effects of nicotinamide appear even with low doses if the duration is adequately long. However, caution is warranted before moving forward with niacin and related compounds for lowering phosphate levels in hemodialysis patients until it is certain that they are effective, well tolerated, and safe for treatment of chronic hyperphosphatemia.
This study was planned to check fasting serum phosphorus; this limited the authors to draw blood for laboratory tests at longer intervals.
ACKNOWLEDGEMENT
This study was granted by Ahvaz Jundishapur University of Medical Sciences. The authors would like to thank the patients for their participation in this study, the faculty of pharmacy for preparing the medications, and Diabetes Research Center staff for their great support.
Table 1. Subjects characteristics and baseline data
Table 2. Within-subjects repeated measures analysis of variance for comparing the changes in variables levels at three stages of measurements (baseline, week 4, and week 8) in nicotinamide group
Table 4. Within-subjects repeated measures analysis of variance for comparing the changes in variables levels at three stages of measurements (baseline, week 4, and week 8) in nicotinamide group for > 75% compliant patients
Figure 1. Flow diagram of participants.
Figure 2. Phosphorus (A), Calcium-phosphorus product (B), HDL (C), and platelet counts (D) changes during study in nicotinamide and placebo groups.
Table 3. Between-subjects repeated measures analysis of variance for comparing the overall changes in variables levels during the study (baseline, week 4, and week 8) in nicotinamide group with those of placebo group