Low-density lipoprotein apheresis (LDL-A), frequently used for peripheral artery disease (PAD) treatment, is expected to induce the improvement of systemic microcirculation.1 Recently, near-infrared spectroscopy was used to evaluate tissue regional oxygen saturation (rSO2) in haemodialysis (HD) patients.2–5 However, there is no report regarding the relation between LDL-A and changes in tissue oxygenation. In our HD patient, we confirmed an improvement in lower-limb muscle oxygenation during LDL-A.
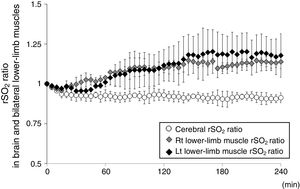
An 82 year-old woman receiving HD was referred to our hospital for treatment of PAD recurrence. Her past medical history included hypertension and insulin-dependent diabetes. HD was initiated beginning 5-years prior. She was diagnosed with PAD and underwent bypass surgery for the right lower leg 4-years ago. Deteriorations of colour of foot skin and foot ulceration were recently confirmed bilaterally in the lower legs; therefore, LDL-A was performed for preventing PAD progression in our dialysis centre. Blood circuits of each LDL-A and HD were tandemly connected: each therapy was simultaneously performed. LDL-A was performed using Plasmaflo OP (Asahi Kasei Medical, Tokyo, Japan) as a plasma separator, a dextran sulfate cellulose column (Liposorba 15, Kaneka, Osaka, Japan) as a LDL absorber, and 1000U/h of heparin sodium as an anticoagulant. Plasma volume was treated at a rate of 50mL/kg per LDL-A session. The duration of LDL-A with HD was 4-h: totally, she received LDL-A once a week for 10 consecutive weeks. During these 10 weeks of LDL-A with HD, other sessions of HD therapy twice a week was performed at a dialysis clinic that she had been receiving that therapy. To confirm LDL-A's influence on microcirculation impairment, tissue rSO2 values in brain and lower-limb muscle were monitored from LDL-A with HD initiation to the end. Written informed consent for rSO2 monitoring during that therapy was obtained. Values of rSO2 were monitored at forehead and bilateral lower-limbs above the gastrocnemii using INVOS 5100c (Covidien Japan, Tokyo, Japan) during second, sixth and 10th sessions of LDL-A with HD. Fluid was removed by ultrafiltration at the level of 1.3±0.4L/session, and haemoglobin levels increased from 9.9±0.4g/dL before LDL-A with HD to 11.0±0.2g/dL after therapy. The rSO2 ratios (mean±standard deviation) in bilateral lower-limb muscles rapidly increased from LDL-A with HD initiation to the end, whereas cerebral rSO2 ratio did not change during that therapy (Fig. 1). Notably, changes in the colour of the foot skin and foot ulceration status improved by LDL-A with HD for 10 consecutive weeks.
The changes in rSO2 of the forehead and bilateral lower-limb muscle as per the oxygenation values of cerebral and muscle tissues, respectively, under LDL-A with HD. rSO2 ratio is defined as the ratio of rSO2 value at t (min) during HD and initial rSO2 value before HD (rSO2 at t (min) during HD/initial rSO2 before HD). The open circle represents the changes in cerebral rSO2 values, the grey diamond shape represents the changes in right lower-limb muscle rSO2 values, and the block diamond shape represents the changes in left lower-limb muscle rSO2 values.
LDL-A has been applied in PAD patients in whom efficacy of conventional pharmacological therapy is insufficient and/or those who are unavailable for surgical therapy because LDL-A itself improves peripheral microcirculation via blood rheology amelioration; reduction of blood and plasma viscosity; production of vasodilating nitric oxide, eicosanoids, and bradykinin; improvement of endothelial function through reduction of total LDL and oxidized LDL concentrations; and reduction of circulating inflammatory cytokines and chemokines.6 Improvement of peripheral microcirculation associated with LDL-A would also be expected to improve tissue oxygenation via increased oxygen supply in peripheral tissues. Indeed, oxygen partial pressure (pO2) in the anterior tibial muscle rapidly and significantly increased during LDL-A in cardiac allograft vasculopathy patients.7 In the present case, bilateral lower-limb muscle oxygenation rapidly improved from LDL-A with HD initiation to the end; our result is consistent with a previous report.7 Ebihara et al. measured systemic blood flow using a laser doppler blood flowmeter during LDL-A, and reported significant increases in tissue blood flow in the head and lower limbs, in which the increase of blood flow was significantly higher in the lower limbs than in the head.1 We did not evaluate the level of tissue blood flow during LDL-A with HD in this case; therefore, we cannot comment on LDL-A-induced blood flow changes in systemic tissues. However, regarding cerebral oxygenation, little changed despite the increase in lower-limb muscle oxygenation; these differences in tissue oxygenation in the brain and lower-limb muscle might be explained by differences in blood flow increase in the head and lower-limb muscle previously reported.1 Additionally, our patient simultaneously received LDL-A and HD therapy; therefore, it could not be definitively determined that the improvement in bilateral lower-limb muscle oxygenation was derived only from the application of LDL-A itself. Regarding tissue oxygenation during HD, there were reportedly no significant changes in cerebral and lower-limb muscle rSO2 values during HD, respectively, although Hb levels after HD increased compared with those before HD.2,8 Therefore, the improvement in muscle oxygenation seen in this case might be caused not by HD therapy but by LDL-A itself.
In conclusion, LDL-A may have improved lower-limb muscle oxygenation in our patient. Thus, it might have positive effects for lower-limb muscle microcirculation.
Conflict of interest statementThe authors have declared that no conflict of interest exists.
We thank the monitored HD patient and dialysis staffs members in our hospital. This work was supported with a grant from the Japanese Association of Dialysis Physicians (No. 27088) provided to S.O., and a grant from The Kidney Foundation, Japan (JKFB16-3) provided to S.O.