Severe congestive heart failure (CHF) patients are prone to hyponatremia. Peritoneal dialysis (PD) is increasingly used for long-term management of refractory CHF patients. The glucose polymer icodextrin was proposed to be a good option for fluid removal in such patients. A small, although statistically significant reduction in serum sodium (∼2mmol/l) consistently observed in multiple trials, is considered as not clinically relevant. Here we reported five refractory CHF patients who demonstrated sodium drop by median of 8meq/l (range 5.4–8.3meq/l) after icodextrin was added to their program. It seems that icodextrin may contribute to clinically relevant hyponatremia if the hyponatremia is compounded by other factors. Patients with extremely severe congestive heart failure are susceptible to this complication.
Los pacientes con insuficiencia cardíaca congestiva grave son propensos a sufrir hiponatremia. La diálisis peritoneal se utiliza cada vez más para el tratamiento a largo plazo de los pacientes con insuficiencia cardíaca congestiva resistentes al tratamiento. El polímero de glucosa icodextrina se propuso como una buena opción para la ultrafiltración. Una reducción pequeña, aunque estadísticamente significativa, del sodio sérico (∼2mmol/l) observada sistemáticamente en numerosos ensayos no se considera de relevancia clínica. En este documento informamos de 5 casos de pacientes con insuficiencia cardíaca congestiva resistentes al tratamiento que presentaron una caída de las concentraciones de sodio en una mediana de 8mEq/l (intervalo 5,4-8,3mEq/l) después de la adición de icodextrina a su programa. Parece ser que la icodextrina puede contribuir a una hiponatremia clínicamente relevante si se combina con otros factores. Los pacientes con insuficiencia cardíaca congestiva muy grave son propensos a esta complicación.
Removal of extensive fluid overload is one of the most difficult challenges in the management of severe congestive heart failure (CHF), particularly in patients who do not respond to diuretic therapy. Peritoneal ultrafiltration (UF) is a simple choice for daily fluid removal. Today, peritoneal dialysis (PD) is increasingly used to treat hypervolemic CHF patients who are resistant to conventional therapies, in particular when complicated by renal insufficiency (reviewed in Refs. 1, 2). Icodextrin was proposed to be a good option for UF in refractory CHF patients.3–6 Icodextrin solution is a long-acting osmotic agent that allows the patient's UF volume to gradually increase for up to 12h.1,3 In a recently published systematic review7 it was found that mortality in CHF patients treated with peritoneal UF was associated with less use of icodextrin.
Icodextrin is generally well tolerated. A small, although statistically significant reduction in serum sodium (∼2mmol/l) consistently observed in multiple trials, is likely not clinically relevant.8 Two female diabetic patients treated by icodextrin-based PD developed severe hyponatraemia with plasma Na<121mmol/l and neurological complications.9 The mechanism by which icodextrin causes hyponatremia is unclear. It was proposed that icodextrin solution leads to accumulation of maltose in the extracellular volume and this gradient induces osmotic flow by a mechanism similar to isosmolar colloid osmosis. The resultant shift in water from the intracellular to the extracellular space can result in dilutional hyponatremia.8 It was demonstrated that icodextrin increased peritoneal Na removal.10
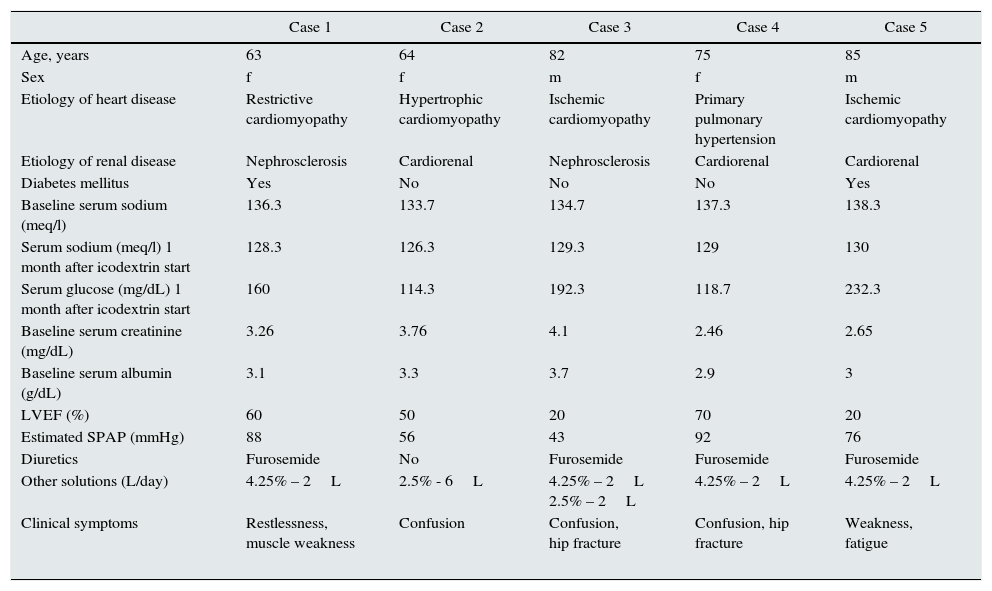
Five refractory CHF patients treated with peritoneal ultrafiltration in our unit, developed significant symptomatic hyponatremia with Na<130mmol/l after icodextrin was added to their program (Table 1).
Cases of severe hyponatremia following icodextrin treatment.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
|---|---|---|---|---|---|
| Age, years | 63 | 64 | 82 | 75 | 85 |
| Sex | f | f | m | f | m |
| Etiology of heart disease | Restrictive cardiomyopathy | Hypertrophic cardiomyopathy | Ischemic cardiomyopathy | Primary pulmonary hypertension | Ischemic cardiomyopathy |
| Etiology of renal disease | Nephrosclerosis | Cardiorenal | Nephrosclerosis | Cardiorenal | Cardiorenal |
| Diabetes mellitus | Yes | No | No | No | Yes |
| Baseline serum sodium (meq/l) | 136.3 | 133.7 | 134.7 | 137.3 | 138.3 |
| Serum sodium (meq/l) 1 month after icodextrin start | 128.3 | 126.3 | 129.3 | 129 | 130 |
| Serum glucose (mg/dL) 1 month after icodextrin start | 160 | 114.3 | 192.3 | 118.7 | 232.3 |
| Baseline serum creatinine (mg/dL) | 3.26 | 3.76 | 4.1 | 2.46 | 2.65 |
| Baseline serum albumin (g/dL) | 3.1 | 3.3 | 3.7 | 2.9 | 3 |
| LVEF (%) | 60 | 50 | 20 | 70 | 20 |
| Estimated SPAP (mmHg) | 88 | 56 | 43 | 92 | 76 |
| Diuretics | Furosemide | No | Furosemide | Furosemide | Furosemide |
| Other solutions (L/day) | 4.25% – 2L | 2.5% - 6L | 4.25% – 2L 2.5% – 2L | 4.25% – 2L | 4.25% – 2L |
| Clinical symptoms | Restlessness, muscle weakness | Confusion | Confusion, hip fracture | Confusion, hip fracture | Weakness, fatigue |
A 63-year-old woman with history of diabetes mellitus (DM), restrictive cardiomyopathy and severe pulmonary hypertension was referred to PD due to significant volume overload. Her eGFR was 27ml/min/1.73m2. She had no proteinuria and renal sonography showed small kidneys. Her kidney disease was attributed to nephrosclerosis with cardiorenal component. She was treated with one dianeal glucose solution 4.25% exchange per day for 6 weeks, then icodextrin was added to her program. She continued the treatment with furosemide and was on sodium restriction diet. Her urinary sodium at icodextrin start was 78meq/l. Following icodextrin start a serum sodium dropped from 136.3 to 128.3meq/l (average of 3 consecutive values) with the lowest value measured of 126meq/l. The patient complained of extreme weakness and fatigue. Icodextrin was stopped and dianeal glucose solution 2.5% was started. After icodextrin stop serum sodium raised to 134.7meq/l (average of 3 consecutive values). Due to difficulty to maintain adequate ultrafiltration and worsening diabetes mellitus control, icodextrin was restarted. Serum sodium dropped to 128.7meq/l (average of 3 consecutive values), lowest value measured was 128meq/l. The patient complained of extreme weakness and fatigue, therefore icodextrin was stopped again. A month later average serum sodium was 133meq/l. The patient died a month later from heart failure exacerbation.
Patient 2A 64-year-old woman with hypertrophic cardiomyopathy was referred to PD due to significant volume overload. She had no proteinuria and demonstrated normal kidneys in renal sonography. Her kidney disease was attributed to cardiorenal syndrome. At PD start the patient's eGFR was 23ml/min/1.73m2. From the very beginning she turned to be hypotensive and oliguric. The patient was 8 months on PD with 3 glucose 2.5% exchanges per day. Furosemide was stopped due to oliguria and hypotension. Urinary sodium was 49meq/l and the patient continued to be edematous. After icodextrin addition serum sodium dropped from 133.7 to 126.3meq/l (average of 3 consecutive values) with the lowest value measured of 125meq/l. The patient continued with icodextrin despite low serum sodium. Non-adherence to free water restriction was suspected. Despite the attempts to restrict free water consumption by the patient serum sodium remained low. The patient died 5 months later from septic shock due to infected cardiac pacemaker electrode.
Patient 3An 82-year-old man with ischemic cardiomyopathy and low LVEF started PD due to resistant volume overload. At the admission his eGFR was 26ml/min/1.73m2. He had non-nephrotic proteinuria and reduced parenchyma size in kidney sonography. His renal disease was attributed to nephrosclerosis with cardiorenal component. He was treated with PD for one month with 2 exchanges per day: glucose 2.5% and glucose 4.25%. He continued treatment with furosemide and was on sodium restriction diet. His urinary sodium was 53meq/l. After icodextrin addition serum sodium dropped from 134.7 to 129.3meq/l (average of 3 consecutive values) with the lowest value measured of 127meq/l. The patient continued with icodextrin despite low serum sodium. He was diagnosed with hip fracture without the history of trauma or fall. He did not recover from the surgery and eventually died in the hospital.
Patient 4A 75-year-old woman with severe primary pulmonary hypertension and cardiorenal syndrome started PD due to resistant volume overload. At the admission her eGFR was 36ml/min/1.73m2. The patient was treated with one glucose 4.25% exchange per day for 2 months. She was edematous therefore icodextrin was added. She continued treatment with furosemide and was on sodium restriction diet. Her urinary sodium was 26meq/l. After icodextrin start serum sodium dropped from 137.3 to 129meq/l (average of 3 consecutive values) with the lowest value measured of 128meq/l. She was reported by her family to be confused. She fell down and broke her hip neck. Icodextrin was stopped during the hospitalization. A month later average serum sodium was 138meq/l (average of 3 consecutive values). The patient eventually died from complications of orthopedic surgery.
Patient 5An 85-year-old man with ischemic cardiomyopathy, low LVEF, severe pulmonary hypertension and diabetes mellitus started with peritoneal UF due to refractory volume overload. His eGFR was 43ml/min/1.73m2 at PD start. He had no proteinuria and normal kidneys in sonography. His kidney disease was attributed to cardiorenal syndrome. He was treated with one glucose 4.25% exchange per day for 3 month, then icodextrin was added. He continued treatment with furosemide and was on sodium restriction diet. His urinary sodium was 31meq/l. After icodextrin start serum sodium dropped from 138.3 to 130meq/l (average of 3 consecutive values) with the lowest value measured of 127meq/l. Icodextrin was stopped. A month later serum sodium was 138.6meq/l (average of 3 consecutive values).
Comparison of severe hyponatremia group with mild hyponatremia groupTo find out which patients were more susceptible to severe hyponatremia after icodextrin addition we compared the characteristics of the above 5 patients as a group (median serum sodium was 129meq/l, range 126.3–130meq/l) with the data of 12 refractory CHF patients who were treated with icodextrin during the same period but their median serum sodium was 134.5meq/l (range 132.3–136.7meq/l). Higher SPAP was more likely connected to significant hyponatremia: median SPAP measured 78mmHg (range 43–92mmHg) in severe hyponatremia group compared to 49mmHg (range 37–67mmHg) in mild hyponatremia group (p=0.0225). Patients who developed significant hyponatremia died earlier compared to patients with insignificant hyponatremia. Median survival was 5 months (range 2–7 months) in severe hyponatremia group compared to 15.5 months (range 6–50 months) in mild hyponatremia group (p=0.0318). There was a tendency for significant sodium drop in women compared to men: in significant hyponatremia group 60% were women compared to 8% women in mild hyponatremia group (p=0.0525). Patients with lower baseline sodium showed a tendency for a more significant sodium drop compared to those with higher baseline sodium. Baseline serum sodium was 136.3meq/l (range 133.7–137.3meq/l) in the severe hyponatremia group compared to 138meq/l (range 134.3–141meq/l) in mild hyponatremia group (p=0.0854).
There was no difference between the groups in NYHA functional class, diabetes mellitus rate, serum creatinine and albumin levels, baseline urinary sodium or diuretics use.
DiscussionWe presented the group of patients who developed clinically relevant hyponatremia following icodextrin addition to the program. Five refractory CHF patients showed serum sodium drop by median of 8meq/l during the first month of icodextrin treatment, more profound decrease than previously reported (Table 1).11–13
It have been proposed that several mechanisms could explain the development of hyponatremia in PD, including net water gain due to excessive water intake or low ultrafiltration rate of free-water, negative Na+/K+ balance caused by low Na+/K+ intake or high Na+/K+ removal, and shift of intracellular water to extracellular space induced by the use of icodextrin or hypercatabolism and malnutrition.14
CHF patients had a tendency to low serum sodium level due to neurohumoral activation. The neurohumoral changes limit both sodium and water excretion in an attempt to return perfusion pressure to normal. ADH release directly enhances water reabsorption in the collecting tubules, whereas angiotensin II and norepinephrine limit distal water delivery by reduction in renal perfusion and by increasing proximal sodium and water reabsorption.15 In addition, both the low cardiac output and high angiotensin II levels are potent stimuli to thirst, leading to enhanced water intake. While significant hyponatremia was temporarily related to icodextrin start, we could not exclude the possibility that hyponatremia in these patients is multifactorial. Probably some our patients were non-adherent to free water restriction, while others demonstrated hyperglycemia (although corrected serum sodium was also low). Other possible contributors to hyponatremia in those patients are dietary restriction and chronic use of diuretics. The risk factors for hyponatremia were female sex, low initial sodium level and increased SPAP. All patients except one had hypertonic glucose 4.25% in their program which could increase thirst and as a result free water consumption. Perhaps, icodextrin metabolites accumulation rises serum osmolality, stimulates thirst and free water consumption which further contribute to hyponatremia in those severe CHF patients.
In our cases patients with severe hyponatremia had lower survival compared to mild hyponatremia group. The studies demonstrated that severe underlying disease worsens hyponatremia and clinical outcome.14 Hyponatremia itself may be a surrogate marker for the severity of underlying disease.14 Hyponatremia is known to be an important marker and prognostic factor of left-sided heart failure.16 Recently, it was demonstrated that hyponatremia was an indicator of poor prognosis in patients with echocardiographic evidence of pulmonary hypertension and preserved left ventricular systolic function.17 Perhaps, patients with certain heart failure etiologies and severe heart disease (as represented by lower survival rate compared to mild hyponatremia group) are more susceptible to this complication.
Two CHF patients who developed significant hyponatremia following icodextrin treatment suffered from hip fracture. There is an emerging literature that suggests that hyponatremia increases the adjusted odds ratio for both falls and fractures in the elderly.18 Hyponatremia appears to contribute to falls and fractures by two mechanisms: it produces mild cognitive impairment resulting in unsteady gait and falls and it directly contributes to bone impairment through osteoporosis due to increased bone resorption in order to mobilize sodium from the bone or altered bone quality through decreased bone microfracture repair.18
ConclusionsHyponatremia in refractory CHF patients is multifactorial. In rare cases, icodextrin may contribute to clinically relevant hyponatremia if the hyponatremia is compounded by other factors. Severe hyponatremia in icodextrin users was associated with poorer survival and is most likely a marker of advanced heart disease. This could be a signal to physician to consider alternative and probably more aggressive interventions.
Conflict of interestsThe authors declare they have no conflict of interest.