Heparin-induced thrombocytopenia (HIT) is a serious and life-threatening complication that occurs in five per cent of patients exposed to heparin. It should be considered in patients with a platelet count <100×109cells/l or a >50% decrease from baseline count in association with heparin therapy. Thromboembolic complications develop in 50% of patients. Bleeding is rare as the platelet count nadir typically does not drop below 20×109cells/l. Up to 12% of dialysis patients develop HIT, named haemodialysis-related-heparin-induced thrombocytopenia (HD-HIT), as they are a risk group with continuous exposure to heparin. The definition of HD-HIT is less strict, in the range of a platelet count decrease of 30% and below 150×109cells/l due to the intermittent use of heparin.
Heparin cessation and alternative anticoagulation are the key interventions in patients with HIT. In dialysis patients, citrate anticoagulation, heparin-free dialysis or peritoneal dialysis are options that must be considered.
The authors describe the presentation, diagnosis, treatment and outcomes of five cases of HD-HIT, and emphasize the importance of an accurate diagnosis and early intervention in order to reduce the mortality risk, which can be as high as 20 per cent.
La trombocitopenia inducida por heparina (TIH) es una complicación grave y potencialmente mortal que aparece en el 5% de los pacientes expuestos a heparina. Debe considerarse en pacientes con una cifra de plaquetas <100×109 células/l o una reducción respecto al valor basal de >50% asociada a tratamiento con heparina. El 50% de los pacientes desarrollan complicaciones tromboembólicas. Se produce hemorragia en raras ocasiones dado que el valor mínimo de la cifra de plaquetas normalmente no desciende por debajo de 20×109 células/l. Hasta el 12% de los pacientes en diálisis desarrollan TIH, denominada trombocitopenia inducida por heparina relacionada con hemodiálisis (TIH-RH), puesto que son un grupo de riesgo con exposición continua a heparina. La definición de TIH-RH es menos estricta, dentro de un rango de descenso de la cifra de plaquetas del 30% y por debajo de 150×109 células/l debido al uso intermitente de heparina.
El cese del tratamiento con heparina y la administración de anticoagulación alternativa son las intervenciones clave en pacientes con TIH. En pacientes en diálisis, la anticoagulación con citrato, la diálisis libre de heparina y la diálisis peritoneal son opciones que deben tenerse en cuenta.
Los autores describen la presentación, el diagnóstico, el tratamiento y los desenlaces de 5 casos de TIH-RH y destacan la importancia de un diagnóstico preciso, así como de una intervención temprana con el fin de reducir el riesgo de mortalidad, que puede alcanzar el 20%.
Heparin-induced thrombocytopenia (HIT) is a serious and life-threatening complication of exposure to heparin products that occurs in five percent of the patients exposed.1,2 Type 1 HIT or non-immune, which affects up to 10% of patients with HIT, is associated with platelet clumping or sequestration due to direct interaction between heparin and circulating platelets, usually within the first 48–72h of drug initiation and only causes mild thrombocytopenia (rarely <100,000/mm3), often returning to normal after heparin withdrawal.3,4
Type 2 HIT or immune mediated is caused by autoantibodies against heparin-platelet factor 4 complexes. It usually occurs within 5–14 days of exposure, but can start abruptly in patients who had exposure to heparin in the previous 90 days, and in these it is sometimes complicated by an anaphylactoid reaction within 30min of administration.3
HIT should be considered when a decrease in the platelet count occurs to less than 100×109cells/L or a fall from baseline count of >50% in association with heparin therapy.2–4 The definition of haemodialysis-related-heparin-induced thrombocytopenia (HD-HIT) is less strict due to the intermittent use of heparin, and diagnosis is made with a platelet count fall of 30% or lower than 150×109cells/L.5–7
Unlike other thrombocytopenic states, it does not induce bleeding but rather results in a prothrombotic state.1,2 An accurate diagnosis and early intervention is essential in order to reduce the mortality risk of this situation, which can reach as high as 20 percent.2,4
The authors describe a case series of five patients diagnosed with HIT and discuss the specificities of the medical approach of HD-HIT which include novel anticoagulants, HD protocol modifications or transition to another renal replacement therapy.
Case seriesFirst caseCaucasian female, 82 years old, hypertensive and with stage 4 chronic kidney disease, was admitted with urinary tract infection due to multiresistant Klebsiella pneumoniae, treated with intravenous Colistin (polymyxin B). Her kidney function declined and she started haemodialysis through a central venous catheter. Fifteen days after starting renal replacement therapy she developed thrombocytopenia, with a nadir platelet count of 33×109cells/L. HIT was suspected, as there was no other cause for thrombocytopenia, 4T score was 5, serological test for HIT antibodies was requested and dialysis anticoagulation was suspended. Citrate was used as the lock in the central venous catheter. Anti-heparin-PF4 antibodies were positive. Within one week of heparin suspension her platelet count was 99×109cells/L. There was no thromboembolic complication. She was discharged for a Haemodialysis Center within two weeks anticoagulated with warfarin.
Second caseAn 83 year-old, Caucasian female, with history of hypertension, atrial fibrillation and stage 5D chronic kidney disease. She had started dialysis the previous month, and after one week developed HIT which resolved with suspension of heparin. She had been started on warfarin but suspended without medical indication. Central venous catheter lock was performed with citrate. She was admitted due to persistent dialysis circuit's clotting complicated with anemia (Hb 7.6g/dL), and thrombocytopenia (Platelet count 12×109). Circuits clotting were interpreted as a consequence of lack of anticoagulation and thrombotic complication of HIT due to previous exposure to heparin. Her 4T's score was 7 with high probability. Serological test for HIT was performed and later revealed to be positive. During hospital stay there were multiple episodes of atrial fibrillation with rapid ventricular response. Her risk for thromboembolic complications was high due to cardiac arrhythmia and HIT. She developed deep venous thrombosis complicated with pulmonary embolism. Anticoagulation with warfarin was re-started. Since then dialysis sessions occurred without complications namely circuit clotting. She was discharged with a platelet count of 100×109cells/L and stable hemoglobin of 10g/dL with indication to maintain anticoagulation with warfarin for 6 months.
Third caseCaucasian female, 73 years old, with stage 3 chronic kidney disease, type 2 diabetes, hypertension, was admitted with septic shock due to urinary tract infection. She had an acute on-chronic kidney injury stage 3 KDIGO; renal function did not improve with resolution of septic shock and she was started on dialysis. Five days after dialysis start platelet count diminished by 50% (220×109→100×109cells/L). Heparin was suspended and central venous catheter lock was performed with citrate. Her calculated 4T's score was 7, and anti-heparin-PF4 antibodies were positive. No anticoagulation was administered during dialysis, and multiple episodes of dialysis circuit's clotting occurred, as well as catheter thrombosis. Platelet count recovery occurred to 153×109cells/L. Warfarin anticoagulation was started. Transition to peritoneal dialysis (PD) was decided, and a peritoneal dialysis catheter was inserted; PD was started eleven days later, complicated by abdominal wall catheter leak. At this moment, partial renal function recovery occurred allowing PD suspension. This partial renal function recovery allowed patient discharge without the need for renal replacement therapy, but with referral to Nephrology follow-up. Platelet count was 180×109cells/L at discharge and she maintained anticoagulation with warfarin for 3 months.
Fourth caseCaucasian male, 85 years old, diagnosed with rapidly progressive renal insufficiency due to propylthiouracil induced vasculitis requiring dialysis. On the seventh day after starting renal hemodialysis he developed thrombocytopenia, with a nadir platelet count of 34×109cells/L. Heparin was suspended and central venous catheter citrate lock was performed. His 4T's score was 4, anti-heparin-PF4 antibodies were positive. Within one week after heparin suspension, platelet count was 150×109cells/L. There was no thromboembolic complication. He was discharged for a Haemodialysis Clinic within two weeks anticoagulated with warfarin.
Fifth caseCaucasian male, 77 years old, with history of multiple myeloma, developed stage 3 KDIGO acute kidney injury and started renal replacement therapy with high cut-off hemodialysis membrane (HCO-HD). Four days after he developed thrombocytopenia with nadir platelet count of 74×109cells/L. Heparin was suspended and central venous catheter citrate lock was performed. His 4T's score was 4 and anti-heparin-PF4 antibodies were positive. Due to the requirement of anticoagulation for HCO-HD, bivalirudin was infused at 0.03mg/kg/h. After 21 days of dialysis he had partial renal function recovery and dialysis was suspended. He had no thromboembolic events. Considering his high risk of bleeding he was discharged without anticoagulation.
In all of these cases other causes of thrombocytopenia were excluded, and heparin induced thrombocytopenia was the most probable cause. Table 1 summarizes these five cases (Fig. 1).
Patient characteristics.
| Patient | History | Onset day/laboratory | Thrombotic events | Therapy | Follow-up |
|---|---|---|---|---|---|
| 82 yo Caucasian female | 12/09/2015 CKD G4→G5D Cause of CKD: Hypertensive nephrosclerosis Cause of AKI: Sepsis+colistin nephrotoxicity HD induction | D15 Hb: 10.4g/dL Nadir Plt: 33×109 4-T's Score: 5 Anti PF4/Hep+ | – | Ø Heparin Citrate lock Warfarin | Plt 99×109 Hemodialysis |
| 83 yo Caucasian female | 26/07/2014 CKD G5D Cause of CKD: diabetic nephropathy HD induction one month ago with HIT Warfarin cessation with HIT | D7 Hb: 7.6g/dL Nadir Plt: 12×109 4-T's Score: 7 Anti PF4/Hep+ | Circuit coagulation Pulmonary embolism | Ø Heparin Citrate lock Warfarin | Plt 100×109 Hemodialysis Death after 2 months (sepsis) |
| 73 yo Caucasian female | 14/04/2015 CKD G3→G5D Cause of CKD: diabetic nephropathy Cause of AKI: septic shock (urinary tract infection) HD induction | D5 Hb: 10.9g/dL Nadir Plt: 100×109 (↓50%) 4-T's Score: 7 Anti PF4/Hep+ | Circuit coagulation | Ø Heparin Citrate lock Warfarin Transition to PD | Plt 180×109 Partial recovery of renal function |
| 85 yo Caucasian male | 16/10/2015 AKI KDIGO3 Cause of AKI: propylthiouracil induced vasculitis HD induction | D7 Hb: 8.3g/dL Nadir Plt: 34×109 4-T's Score: 4 Anti PF4/Hep+ | – | Ø Heparin Citrate lock | Plt 150×109 Hemodialysis |
| 77 yo Caucasian male | 05/12/2014 AKI KDIGO3 Cause of AKI: Light-chain nephropathy (multiple myeloma) HD induction | D4 Hb: 9.1g/dL Nadir Plt: 74×109 4-T's Score: 4 Anti PF4/Hep+ | – | Ø Heparin Citrate lock Bivalirudin | Plt 180×109 Partial recovery of renal function |
Yo: years old; AKI: acute kidney injury; CKD: chronic kidney disease; Hb: hemoglobin; Plt: Platelets
Heparin-induced thrombocytopenia (HIT) is the most frequent drug-induced type of thrombocytopenia and it is associated with significant morbidity and mortality if unrecognized.2,4
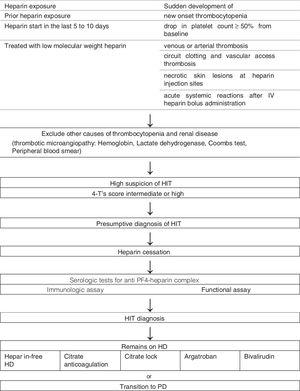
It is important to suspect HIT in patients with prior heparin exposure, who began heparin in the last 5–10 days or receiving prolonged treatment with low molecular weight heparin, that suddenly develop new onset thrombocytopenia, a drop in platelet count of more than 50% from baseline, venous or arterial thrombosis, necrotic skin lesions at heparin injection sites or acute systemic reactions after IV heparin bolus administration (Table 2).3,4,8
4-T's score.
| Category | 2 points | 1 point | 0 points |
|---|---|---|---|
| Thrombocytopenia | Platelet count falls by >50% and platelet nadir ≥20×10/l | Platelet count falls by 30–50% or platelet nadir 10–19×109/l | Platelet count falls by <30% or platelet nadir <10×109 |
| Timing | Occurs between days 5–10 or in less than 1 day if previous exposure in the last 30 days | Onset after day 10 or fall in less than one day if exposure in last 30–100 days | Early fall of platelets with no previous exposure |
| Thrombosis or other sequelae | New thrombosis, skin necrosis, post-heparin bolus acute systemic reaction | Progressive or recurrent thrombosis; erythematous skin lesions, suspected thrombosis not yet proven | None |
| Other cause for thrombocytopenia | No other cause is evident | Possible cause is evident | Definite other cause is evident |
Thrombocytopenia is the most common manifestation, presenting in 85–90% of patients and results from the removal of IgG coated platelets and consumption of activated platelets within thrombi.4 In 25% of the patients thrombosis is the presentation form. Venous thrombosis is more common than arterial thrombosis.4,9 Pulmonary embolism is the most common life-threatening event.4 Thromboembolic complications are a result of increased thrombin produced by activated platelets and monocytes, endothelial cell activation and injury. Circuit clotting and vascular access thrombosis are a common manifestation of HIT in dialysis patients, but other causes of access thrombosis must be excluded.9
HIT can occur regardless of dose, schedule and administration route.1 The heparin-platelet 4 complexes form most efficiently with unfractioned heparin, less efficiently with low molecular weight heparin and are negligible with fondaparinux.1,4 It is more common in women, older age and surgical patients.4
Due to continuous exposure to heparin, dialysis patients are a risk group, and up to 12% develop HIT.5 HIT usually occurs in an early session after starting dialysis with heparin, however some patients with the PF4/heparin complex antibody may have a risk of delayed-onset HIT.7 The clinical presentation of HD-HIT includes acute thrombocytopenia, clotting of the extracorporeal circulation undergoing heparin administration and disappearance of clots on using an alternative anticoagulant, and rarely, thrombotic occlusion in vascular access.5,7
All patients with presumptive diagnosis of HIT should have laboratory testing for HIT antibodies (anti-PF4-heparin) but these results should not delay intervention.1–3 Presumptive diagnosis is based on clinical findings and available laboratory data.2 The 4 T's score quantifies the clinical findings associated with HIT and scores the degree of thrombocytopenia, timing, thrombotic events or sequelae, and alternative causes of thrombocytopenia10 (Table 2). If the 4 T's score is intermediate or high probability, we make a presumptive diagnosis of HIT.10
The laboratory tests to confirm HIT include immunologic and functional assays. Immunologic assays, such as the enzyme-linked immunosorbent assay (ELISA), are highly sensitive and can be used to exclude HIT.11,12,15 By measuring the optical density, restricting antibody detection to the IgG subclass or repeating the assay in high heparin concentrations the specificity of these tests can be increased12,13 Functional assays, such as the serotonin-release assay and heparin-induced platelet activation test), are much more specific for HIT.14,15 Diagnostic accuracy is increased by combining immunologic assays, that detect antibodies, and functional assays, that detect platelet activation.14,15 The Platelet Immunology SSC Working Group on HIT recommends that the diagnosis of HIT is made by the clinical presentation in which HIT is judged to be the most plausible diagnosis, a scoring system with at least an intermediate probability for HIT, plus either a strong-positive EIA-IgG or a positive test for platelet-activating antibodies.15
We highlight the importance of an accurate diagnosis; in fact, the risks of misdiagnosing HD-HIT concern not only underuse of anticoagulation in patients with thrombotic risk, but also the hemorrhagic risk, as well as costs, related to inappropriate use of anticoagulation. Also, it is important to consider the risk of an inadequate dialysis dose delivered due to repeated episodes of clotting of the extracorporeal circulation, emphasizing the need of an early diagnosis and therapeutic intervention to minimize this risk.
These patients should have immediate discontinuation of heparin of all sources and administration of a non-heparin anticoagulant, such as argatroban, danaparoid, fondaparinux or bivalirudin, due to cross-reactivity between heparin products, and persistent risk of thrombosis even after thrombocytopenia resolves.11,16,17 However, if there is bleeding or significant risk of bleeding, anticoagulation might be contraindicated.
Fondaparinux is the first line of anticoagulation in patients with liver failure, and can be used if there is the need for a subcutaneous agent, however it is not indicated in cases of renal failure, in which argatroban and bivalirudin are the preferable agents, as well as for patients with combined hepatic and renal failure.5,6,17
Argatroban is metabolized in the liver and does not require adjustment according to renal function, and is infused at 2mcg/kg/min or 0.5mcg/kg/min in patients with liver failure.9,16–18 Bivalirudin has liver and renal clearance, it is infused at 0.15mg/kg/h, 0.14mg/kg/h in patients with liver failure, 0.03–0.05mg/kg/h in patients with renal or combined liver and renal failure, and 0.04–0.03mg/kg/h in patients receiving continuous renal replacement therapy (Table 3).16–20
Anticoagulants used for dialysis patients.
| Anticoagulant | Dose |
|---|---|
| Argatroban | 2mcg/kg/min 0.5mcg/kg/min in patients with liver failure |
| Bivalirudin | 0.15mg/kg/h 0.14mg/kg/h in patients with liver failure 0.03–0.05mg/kg/h in patients with renal or combined liver and renal failure 0.04–0.03mg/kg/h in patients receiving continuous renal replacement therapy |
Both are direct thrombin inhibitors, and their effect is monitored with aPTT, which should be maintained at one and a half to three times the normal value. Argatroban does not show cross-reactivity with HIT antibodies, and patients exposed to bivalirudin have a very low incidence of developing drug-induced antibodies.9,16–20
Rivaroxaban is beginning to be considered as an alternative agent in treatment of HIT but further data is needed for definitive approval.3,4,7,16,17
Warfarin should only be used after the patient is anticoagulated with an alternative anticoagulant, platelet count is above 150×109cells/L and there should be an overlap period of 5 days minimum before alternative anticoagulant is stopped, as the risk of thrombosis with warfarin alone is markedly increased.2,4,16,17,21
The resolution of thrombocytopenia should occur within seven days after withdrawal from heparin. The length of anticoagulation is not defined, however patients should continue anticoagulation with a non-heparin anticoagulant for at least three months, during which anti-PF4 antibodies are still in circulation, and for at least six months if a thrombotic event has occurred.8,16,17,21,22 Re-exposure to heparin should be avoided as these patients have risk of developing thrombocytopenia or recurrence of HIT, thus alternative anticoagulation should be considered.16,17,21,22
For dialysis patients heparin discontinuation of all sources includes low-molecular-weight-heparin, heparin flushing, and heparin-coated catheters or devices.5–7 Argatroban and bivalirudin are the preferred alternate method of anticoagulation for dialysis patients.9,10 Other therapeutic methods can also be employed such as heparin-free dialysis and peritoneal dialysis (Fig. 1).6,7,9
Although small, our one-year cohort of HD-HIT patients reflects a slight female preponderance, patients older than 70 years, as described in the literature, presenting soon after dialysis was initiated. Heparin was promptly suspended as soon as HIT was suspected, and allowed for platelet count recovery in all patients. The laboratory test in our hospital is the ELISA immune-assay, which is used only for highly suspicious cases as an exclusion test. The second case demonstrates the importance of alternate anticoagulation due to the risk of thromboembolic complications. These cases demonstrate the complexity of therapeutic approach to these patients. Alternate anticoagulation with warfarin was initiated in four of the five patients; however, bridging warfarin therapy with alternate anticoagulation agent was not performed due to unavailability by the time these events occurred (only fondaparinux was available, which is contraindicated in dialysis patients). In the last case bivalirudin was used as anticoagulant during HCO-HD, which had recently been introduced at our hospital. Furthermore, alternate methods were useful such as heparin free dialysis with citrate catheter lock, since all patients had catheter with heparin lock, and peritoneal dialysis.
The high prevalence of comorbidities of the described patients raises the importance of proper medical approach of HD-HIT. Although a HD-HIT is prothrombotic situation, account must be taken that renal failure patients are also more prone to hemorrhagic events under anticoagulation; in fact, simple issues as the safe dose of low-molecular weight heparin for dialysis patients is not well established.14,16,17,22
ConclusionIn conclusion, HD-HIT must be suspected in a dialysis patient receiving heparin who present with thrombocytopenia in the first five to fourteen days after heparin exposure. Each patient must have an individualized approach, although heparin and heparin products must be immediately stopped before a presumptive HD-HIT diagnosis. Concerning renal replacement therapy and anticoagulant therapy individualized approach must be made considering the thrombotic risk of this situation.
FundingThis research received no funding from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors declare no conflict of interests.
None.