Chronic kidney disease (CKD) is a growing health problem. About 20% of CKD patients have undetermined causes. Data describing histopathological patterns of unexplained impaired kidney functions in Egypt are lacking. We aimed to identify the clinicopathological characteristics and short-term outcomes of adult cases with unexplained impaired kidney functions.
MethodsWe conducted a prospective study in Assiut University Hospital from August 2018 to May 2022. It included patients with unexplained elevated serum creatinine (serum creatinine>115μmol/L) who underwent renal biopsies. Descriptive statistics were used to summarize data on patient demographics, histopathological patterns, and outcomes after three months. Clinical trial registration number: NCT03586531.
ResultsOverall, 210 native renal biopsies were included in the analysis. Glomerular diseases were the most common pathological finding (n=88, 44.9%), amyloidosis and FSGS were the most prevalent glomerular pathology (15.2%, 14.3%, respectively). Chronic kidney disease of unknown etiology (CKDu) was diagnosed in 8.1% of the cases; histology suggestive of genetic origin was found in 2.5%, and LECT amyloidosis was found in 3.8% of the cases. Poor outcomes were observed in 43.6% of the patients i.e., renal replacement therapy or death. Treatment strategies were changed based on the biopsy findings in 86 patients (40%).
ConclusionAmyloidosis and FSGS were the most common causes of unexplained renal impairment. CKDu is not uncommon in Egypt, and more preventive measures are needed. This study supports the irreplaceable role of renal biopsy in disease diagnosis, treatment decisions, and predicting prognosis even in advanced stages.
La enfermedad renal crónica (ERC) es un problema de salud en crecimiento. Aproximadamente el 20% de los pacientes con ERC tienen causas no determinadas. Los datos que describen los patrones histopatológicos de la disfunción renal inexplicada en Egipto son escasos. Nuestro objetivo fue identificar las características clinicopatológicas y los resultados a corto plazo de casos adultos con disfunción renal inexplicada.
MétodosRealizamos un estudio prospectivo en el Hospital Universitario de Assiut desde agosto de 2018 hasta mayo de 2022. Se incluyeron pacientes con niveles elevados inexplicados de creatinina sérica (creatinina sérica>115μmol/l) que se sometieron a biopsias renales. Se utilizaron estadísticas descriptivas para resumir los datos sobre la demografía de los pacientes, los patrones histopatológicos y los resultados después de tres meses. Número de registro del ensayo clínico: NCT03586531.
ResultadosEn total, se incluyeron 210 biopsias renales nativas en el análisis. Las enfermedades glomerulares fueron el hallazgo patológico más común (n=88, 44,9%), siendo la amiloidosis y la glomeruloesclerosis focal y segmentaria (FSGS) las patologías glomerulares más prevalentes (15,2% y 14,3%, respectivamente). La enfermedad renal crónica de causa desconocida (ERCn) se diagnosticó en el 8,1% de los casos; la histología sugerente de origen genético se encontró en el 2,5%, y la amiloidosis LECT se detectó en el 3,8% de los casos. Se observaron malos resultados en el 43,6% de los pacientes, es decir, terapia de reemplazo renal o muerte. Las estrategias de tratamiento se modificaron en 86 pacientes (40%) basándose en los hallazgos de la biopsia.
ConclusiónLa amiloidosis y la FSGS fueron las causas más comunes de insuficiencia renal inexplicada. La ERCn no es infrecuente en Egipto, y se necesitan más medidas preventivas. Este estudio respalda el papel insustituible de la biopsia renal en el diagnóstico de enfermedades, las decisiones de tratamiento y la predicción del pronóstico, incluso en etapas avanzadas.
Chronic kidney disease (CKD) is a growing healthcare problem that affects more than 800million people worldwide. Its rank is projected to advance from the 16th to the 5th cause of death globally by 2040.1 CKD is mostly caused by diabetes mellitus (DM), hypertension (HTN), and glomerular diseases. However, sometimes the etiology of CKD is unknown owing to the absence of identifiable risk factors, or specific histological findings.2 This condition is called chronic kidney disease of unknown etiology (CKDu).3
In a 2006 study, CKDu was reported in 27% of patients who received dialysis in El-Minia Governorate of Egypt; however, their diagnosis was not based on histological findings.4 It was uncertain if the patients developed their condition after exposure to risk factors related to the agricultural community or if they presented late after having undiagnosed treatable conditions. A definite diagnosis can be achieved through renal biopsy, especially when conventional workup is inconclusive. Renal biopsies also provide prognostic information for renal and patient survival and can help in deciding treatment options and pre-transplant workup, even in advanced cases.5
In an Egyptian study, researchers analyzed the clinic pathological data of patients who underwent renal biopsy at their center in 2020. They found that 47.8% of the biopsies were performed for patients with accidentally discovered renal impairment.6 However, to date, there is no Egyptian registry for data on the pathological results of renal biopsies in patients with unexplained renal impairment. Identifying the underlying pathology in these patients could help healthcare professionals provide optimum and timely therapeutic options and preventive measures for their condition. Herein, we aimed to identify the histopathological pattern of patients with unexplained renal impairment, their clinical outcomes, and the clinic pathological characteristics of patients with CKDu.
Materials and methodsStudy designThis prospective observational study was conducted at the Nephrology Unit of Assiut University Hospital, which provides tertiary care for patients from Upper Egypt. It included adult patients with renal impairment that could not be diagnosed with clinical and laboratory parameters (unexplained renal impairment). Patients were recruited between August 2018 and May 2022 and followed up for three months after the date of their biopsy. The study protocol was approved by the Faculty of Medicine Ethical Review Board at Assiut University (Approval number: IRB17200010), and registered in clinicaltrials.gov, registration number: NCT03586531. The study was performed in accordance with the principles of the Declaration of Helsinki.
PatientsInformed consent was obtained from all individual participants included in the study. All patients fulfilled the following inclusion criteria: age≥18 years old, elevated serum creatinine>115μmol/L, negative serological markers (ANA, antidsDNA, perinuclear or cytoplasmic antineutrophil cytoplasmic antibodies, and normal complement (C3 and C4)) levels. Patients with the following criteria were excluded from the study: diabetes with clinical and laboratory data favoring diabetic nephropathy,7 hypertension with clinical data suggestive of hypertensive nephrosclerosis,8 suspicion of renal involvement in systemic disease, history of nephrotoxic drugs, the presence of relative or absolute contraindications for the procedure, kidney size<8cm, and pregnancy.
MethodsPatients’ data were collected at the biopsy visit and included demographics, detailed medical and medication history, and essential laboratory investigations. Serum creatinine was measured using a Jaffe-based method, and urine proteins were measured by an immunoturbidometric method. GFR was estimated using the creatinine-based Chronic Kidney Disease Epidemiology Collaboration equation.
Biopsy procedureBiopsies were done by skilled nephrologists according to standard procedures.9 Biopsy complications were categorized as minor and major complications. Minor complications included flank pain, hematuria, and/or spontaneously resolving hematoma without the need for further intervention. Major complications were those that resulted in hemodynamic instability, need for an intervention, such as transfusion of blood products, further imaging, invasive radiologic or surgical procedures, or death.
All biopsies were processed routinely for light microscopy and immunohistochemistry and examined by one of two experts in nephropathology. Immunoperoxidase (IP) studies were performed using antihuman IgG, IgA, IgM, C3, kappa, and lambda light chains. Cases were examined by electron microscopy (EM) when feasible based on the findings of clinical examination.
TreatmentTreatment plans were either “specific treatment,” which were dependent or modified according to the histological diagnosis, or “supportive treatment” due to the presence of advanced lesions or the unavailability of specific treatment.
Patients’ outcomesPatients were followed for 3 months for the following end points: renal recovery (creatinine return to/or within 25% of the baseline value and dialysis independence),10 need for maintenance dialysis, or death from causes not related to the biopsy procedure.
StatisticsData analysis was performed using SPSS version 26.0 (SPSS Inc., Chicago, IL, US). Means±SD were used to describe normally distributed variables, while medians and ranges were used to describe non-normally distributed variables. Chi-square/Fisher Exact tests were used to compare proportions between groups. One way ANOVA or Kruskal–Wallis tests were used to compare mean and median differences between groups. A post hoc test for pairwise comparison with Bonferroni correction was done to identify significance between more than two groups. Univariate logistic regression analysis was performed to identify possible predictors for poor outcomes among patients, and significant variables were entered in a multivariate backward Wald logistic regression analysis to calculate adjusted odds ratios (AOR). Statistical significance was set at p<0.05.
ResultsDuring the study period, 210 of 687 native biopsies (30.6%) were performed for unexplained renal impairment. Two were excluded from the analysis due to inadequate sampling (medulla only). The patients were mostly middle-aged with near equal sex distribution. Their mean serum creatinine level was seven folds higher than normal. The most frequent comorbidities were hypertension followed by diabetes. Post-biopsy complications occurred in 19 patients. Six patients developed major complications in the form of large perinephric hematoma necessitating blood transfusion. Five of these patients were managed conservatively, and one underwent super-selective renal artery angioembolization to control bleeding. The other 13 patients had minor complications as a small perinephric hematoma (five patients) and gross hematuria (eight patients) (Table 1).
Demographic and clinical data of the studied participants (n=210).
| Demographic data | |
| Age, year | 43.7±14.9 |
| <40 | 88 (41.9%) |
| 40 to <65 | 95 (45.2%) |
| ≥65 | 27 (12.9%) |
| Male | 107 (51%) |
| Current smoking | 51 (24.3%) |
| Substance abuse | 23 (11%) |
| Farmer | 51 (24.3%) |
| Medical history | |
| Hypertension | 64 (30.5%) |
| DM | 19 (9%) |
| Cardiac diseases | 6 (2.9%) |
| Chronic kidney disease | 5 (2.4%) |
| Thyroid diseases | 4 (1.9%) |
| COPD | 1 (0.5%) |
| Clinical data | |
| SBP, mmHg | 134±14.9 |
| DBP, mmHg | 83.2±8.3 |
| MAP, mmHg | 100.1±9.8 |
| Nephrotic syndrome | 46 (21.9%) |
| Creatinine, μmol/l | 724 (136–2300) |
| Microscopic hematuria | 88 (41.9%) |
| 24-h protein, mg/day | 1217 (117–9700) |
| eGFR (ml/min/m2)a | 6 (2–50) |
| Lt kidney length, cm | 10.1±1.4 |
Data are expressed as percentages, (mean±SD), or (median, range) as appropriate.
COPD: Chronic Obstructive Pulmonary Disease, eGFR: estimated glomerular filtration rate, DBP: diastolic blood pressure, DM: diabetes mellitus, MAP: mean arterial blood pressure, SBP: systolic blood pressure.
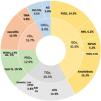
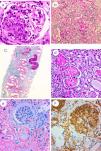
Patients were categorized according to their histological diagnosis into five groups: glomerular, tubulointerstitial, vascular, paraprotein-related renal disease (PRDs), and pathology suggestive of CKDu (Fig. 1). The most frequent diagnoses were glomerular and tubulointerstitial diseases, accounting for two-thirds of all biopsies. The most frequent glomerular pathologies were amyloidosis (n=32, 15.2%) and focal segmental glomerulosclerosis (FSGS, 14.3%). In the amyloidosis group, 14 patients had ALECT2; 18 patients had AA amyloidosis; and two patients had AL amyloidosis (categorized as PRDs). Within the tubulointerstitial disease (TIDs) group, five patients (2.4%) had histological criteria highly suggestive of genetically inherited diseases: autosomal dominant tubulointerstitial kidney disease (ADTKD) in three patients, and nephronophthisis in two patients. Eight patients had chronic tubulointerstitial nephritis (3.8%), of whom six had granulomatous nephritis, and two had IgG4 tubulointerstitial nephritis. PRD comprised 12.9% of cases, with light chain cast nephropathy as the predominant type, comprising 10.5% of all cases. Some histological diagnoses are shown in Fig. 2.
Histological groups and subgroups of the studied groups. AG: advanced glomerulosclerosis, AIN: acute interstitial nephritis, AL: light chain amyloidosis, ATN: acute tubular necrosis, Cast N: cast nephropathy, CKDu: chronic kidney disease of undetermined etiology, CNS TIN: chronic non-specific tubulointerstitial nephritis, CTIN: chronic tubulointerstitial nephritis, FSGS: focal and segmental glomerulosclerosis, GDs: glomerular diseases, MIDDs: monoclonal immunoglobulin deposition disease; MN: membranous nephropathy, MPGN: membranoproliferative pattern glomerulonephritis, NGN: necrotizing glomerulonephritis, PRDs: paraprotein-related renal diseases, TIDs: tubulointerstitial diseases, TMA: thrombotic microangiopathy, VDs: vascular diseases.
Some histological diagnoses. (a) S: under light microscopy using H&E stain showing a segmental area of collapsed capillaries with increased mesangial matrix (original magnification ×200). (b) Picture suggestive of CKDu showing chronic interstitial nephritis, marked fibrosis, and sclerosed glomeruli by PAS stain (original magnification ×200). (c) Picture suggestive of CKDu showing diffuse atrophic changes with mild lymphocytic infiltrate on background of diffuse interstitial fibrosis by Masson trichrome stain (original magnification ×100). (d) Cast nephropathy by H&E, tubules showed moderate injury with large fractured PAS negative hyaline cast surrounded by tubular epithelial cells, inflammatory cells and few giant cells (original magnification ×200). (e) LECT amyloidosis glomerular and interstitial congo red uptake (original magnification ×200). (f) Anti-LECT2 antibody positive reactivity of glomeruli and interstitial deposit (immunoperoxidase technique original magnification ×200).
We compared the five main histological groups as regards their clinical variables and compared each histological group with the CKDu group. The youngest patients were in the CKDu group, and the oldest patients were in the PRDs group, with a mean age of 37.8±13.6 vs. 50.5±9.7 years (p=0.006), respectively. The proportion of males was highest in the CKDu group (11/17, 64.7%) and lowest in the TIDs group (11/17, 64.7% vs. 15/43, 34.9%; p=0.036, respectively). The greatest proportion of patients working as farmers were in the CKDu group and comprised 47.1% of patients (n=8, p=0.001) (Table 2).
Demographic and clinical characteristics of patients who underwent renal biopsy by histological diagnosis.
| Variables | CKDu, n=17 | GDS, n=88 | TIDs, n=43 | PRDs, n=27 | VDs, n=35 | p-Value* |
|---|---|---|---|---|---|---|
| Age, ys | 37.8±13.6 | 44.7±14.6 | 40.2±16. | 50.5±9.7** | 43.1±16.3 | 0.024 |
| Male | 11 (64.7%) | 53 (60.2%) | 15 (34.9%)** | 12 (44.4%) | 16 (45.7%) | 0.048 |
| Farmer | 8 (47.1%) | 27 (30.7%) | 1 (2.3%)** | 7 (25.9%) | 8 (22.9%) | 0.001 |
| Current hypertension | 13 (76.5%) | 61 (69.3%) | 16 (59.3%) | 23 (53.5%) | 21 (60.0%) | 0.310 |
| Nephrotic syndrome | 1 (5.7%) | 33 (37.5%)** | 0 (0%) | 5 (18.5%) | 7 (20%) | <0.001 |
| SBP, mmHg | 140.9±10.4 | 135.9±16.1 | 130.7±14.1** | 134±14.2 | 141.3±16.4 | 0.070 |
| Creatinine (μmol/l) | 725 (262–2082) | 638.5 (145–2300) | 975 (211–1767) | 765 (136–1641) | 788 (197–2000) | 0.044 |
| eGFR ml/min/m2a | 7 (2–28) | 7 (2–50) | 7 (2–36) | 5 (2–23) | 5 (2–39) | 0.107 |
| iPTH, pg/ml | 300 (50–1900) | 214 (9–1652)** | 168 (30–1621)** | 156 (11–949)** | 189 (58–798)** | 0.236 |
| Albumin, g/L | 29.8±7.1 | 31.3±6.7 | 31.9±6.6 | 28.1±5.8 | 26.5±8.8 | 0.058 |
| ALP, U/L | 138.6 (78–304) | 81.5 (36–176) | 79 (29–370) | 89 (46–389) | 115 (57–214) | 0.108 |
| TP, g/L | 60.6±7.6 | 58.4±9.5 | 64.6±9.9 | 59.2±9.4 | 66.6±12.8 | 0.024 |
| U. ptn, g/24h | 0.8 (0.27–4.33) | 2.31 (0.12–9.7)** | 0.53 (0.12–3.47)** | 1.01 (0.15–4.7) | 1.52 (0.15–5.22) | <0.001 |
| Microscopic hematuria | 3 (17.6%) | 35 (39.8%) | 15 (34.9%) | 9 (33.3%) | 26 (74.3%)** | <0.001 |
| Lt kidney, cm | 9.48±0.79 | 9.88±1.10 | 10.03±1.21 | 10.50±1.52** | 10.09±1.25 | 0.065 |
ALP: alkaline phosphatase, CKDu: chronic kidney disease of unknown etiology, eGFR: estimated glomerular filtration rate, GDS: glomerular diseases, Hgb: hemoglobin level, iPTH: intact parathyroid hormone, PRDs: paraprotein-related renal diseases, SBP: systolic blood pressure, TIDs: tubulointerstitial diseases, TP: total protein, VDs: vascular diseases.
eGFR was estimated by CKD-EPI equation.
Data were expressed as frequency (percentage), Mean±SD, or median (range).
Forty-six patients (21.9%) presented with renal impairment and nephrotic syndrome, unsurprisingly most of them had glomerular diseases and none of them had TIDs (Table 2).
The lowest mean serum creatinine level was observed in the glomerular disease group (638.5μmol/L), while the highest level was observed in the tubulointerstitial diseases group (975μmol/L), and the difference was statistically significant (Table 2).
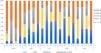
The median iPTH was high in all groups, with a statistically significant difference between the CKDu group and other groups. Sixty percent of the patients presented with sub-nephrotic range proteinuria (Fig. 3).
Advanced chronic changes, such as glomerular sclerosis, tubular atrophy and interstitial fibrosis were more frequently noted in the CKDu group compared with other groups (p=0.033, p=0.002, and p<0.001, respectively) (Fig. 4).
Histopathological findings of the studied groups. CKDu: chronic kidney disease of unknown etiology, GS: glomerulosclerosis, IF: interstitial fibrosis, PRDs: paraprotein-related renal diseases, TA: tubular atrophy, TIDs: tubulointerstitial diseases, VDs: vascular diseases. P1 comparison between glomerulosclerosis among whole groups by Chi-square test, P2 comparison between tubular atrophy among whole groups by Chi-square test, P3 comparison between interstitial fibrosis among whole groups by Chi-square test, P4 comparison between arterial sclerosis among whole groups by Chi-square test. AS: arterial sclerosis, GDs: glomerular diseases.
Patients with PRDs or VDs were more likely to be assigned specific treatment based on their biopsy results. However, all patients in the CKDu group needed only supportive treatment, including renal replacement therapy (Fig. 5).
The patients were followed for 3 months for a more consistent definition for renal disease and proper CKD stages. The most frequent outcome among CKDu and glomerular disease groups was the need for maintenance dialysis. Most of the patients who recovered were in the TIDs group. Three patients died within the follow-up time frame from causes unrelated to the biopsy procedure (Table 3).
patient's outcome by histological group.
| Variables | All cohort, N=210 | CKDu, n=17 | GDs, n=88 | PRDs, n=27 | TIDs, n=43 | VDs, n=35 |
|---|---|---|---|---|---|---|
| Improved | 43 (20.5%) | 0 (0.0%) | 7 (8.0%) | 6 (22.2%) | 26 (60.5%) | 4 (11.4%) |
| CKD ND | 76 (36.2%) | 3 (17.6%) | 37 (42%) | 8 (29.6%) | 9 (20.9%) | 19 (54.3%) |
| CKD-D | 88 (41.9%) | 14 (82.4%) | 44 (50.0%) | 11 (40.7%) | 8 (18.6%) | 11 (31.4%) |
| Death | 3 (1.4%) | 0 (0.0%) | 0 (0.0%) | 2 (7.4%) | 0 (0.0%) | 1 (2.9%) |
Values represent frequency (percentage).
CKD-D: chronic kidney diseases on dialysis, CKD-ND: chronic kidney diseases not on dialysis, CKDu: chronic kidney disease of unknown etiology, GDS: glomerular diseases, PRDs: paraprotein-related renal diseases, TIDs: tubulointerstitial diseases, VDs: vascular diseases.
Univariate and multivariate regression analysis were performed to determine which factors were associated with poor outcomes (death and need for maintenance dialysis). A univariate model showed that multiple factors were significantly associated with poor outcomes (Table 4). However, in the multivariate model, only higher serum urea, high systolic blood pressure (SBP), and lower estimated glomerular filtration rate were associated with inferior outcomes. Severe glomerulosclerosis (GS) and interstitial fibrosis (IF) were significantly associated with inferior outcomes, with the highest adjusted odds ratio for severe IF (adjusted odds ratio [AOR], 6.41[2.19–18.76], p 0.001).
The variables associated with poor outcome on univariate and multivariate analysis.
| Predictors | Univariate | p-Value | Multivariate | p-Value |
|---|---|---|---|---|
| OR (95% C.I.) | AOR (95% C.I.) | |||
| Age | 0.99 (0.97–1.01) | 0.519 | ||
| Urea | 1.05 (1.02–1.07) | <0.001 | 1.03 (1.01–1.06) | 0.025 |
| Creatinine | 1.02 (1.001–1.03) | <0.001 | ||
| iPTH | 1.03 (1.002–1.05) | <0.001 | ||
| eGFR EPI | 0.88 (0.83–0.93) | <0.001 | 0.90 (0.84–0.97) | 0.006 |
| SBP | 1.027 (1.01–1.05) | 0.007 | 1.03 (1.01–1.05) | 0.019 |
| MAP | 1.03 (1.01–1.06) | 0.039 | ||
| 24h protein | 1.00 (1.00–1.00) | 0.097 | ||
| GS≥50% | 5.68 (2.79–11.56) | <0.001 | 2.63 (1.05–6.60) | 0.040 |
| IF≥50% | 10.19 (4.26–24.35) | <0.001 | 6.41 (2.19–18.76) | 0.001 |
| Marked TA | 4.07 (1.93–8.53) | <0.001 | ||
| AS | 2.48 (1.42–4.35) | 0.001 |
Model used was backward Wald logistic regression.
p value significant<0.05 and shown in bold.
AOR: adjusted odds ratio, As: arterial sclerosis, CI: confidence interval, eGFR: estimated glomerular filtration rate, GS: glomerulosclerosis, IF: interstitial fibrosis, iPTH: intact parathyroid hormone, MAP: mean blood pressure, OR: odds ratio, SBP: systolic blood pressure, TA: tubular trophy.
In this study, we investigated the histopathological findings of renal biopsies in patients with unexplained renal impairment. We found that glomerular diseases were the most common histological diagnosis and that severe GS and IF were significantly associated with inferior outcomes.
Every geographical area has a unique renal disease profile that becomes more evident with histopathological analysis. In our study, we found the most common histological diagnosis was glomerular diseases (41.9%). This was in agreement with Mittal et al., who found that 60% of patients presenting with CKD of undetermined etiology in Northern India had glomerular diseases.11 This finding may reflect a delay in diagnosis as the majority of patients presented in advanced stages. Moreover, the median creatinine in our patients was 724μmol/L, which was much higher than that reported by Syahril et al. (306μmol/L) who investigated the renal histology in patients presenting by unexplained elevated serum creatinine in Malaysia. This reflects the problem of delayed referral to nephrologists in our region.12
The leading pathologies for glomerular diseases were FSGS (14.3%) and amyloidosis (11.4%). Similarly, in a study by Mittal et al., FSGS was the leading primary glomerulonephritis accounting for 18.2% of the cohort.11 In contrast, Zaza et al. found that the leading types of glomerulonephritis detected from renal biopsies in Italian patients with CKD were IgA (24.3%) nephropathy and FSGS (13.3%).13 Nevertheless, we did not recognize cases of IgA nephropathy- among our patients. This may be explained by the lower prevalence of IgA in Africa.14
A definite cause for renal impairment could not be determined in 10.4% of the cohort. This was close to that reported in previous studies15,16 and lower than reported by Titze et al.17 Of them, 2.3% had clinical i.e., positive family history, positive consanguinity, or being young adults, and tubulointerstitial findings favoring a diagnosis of genetic origin i.e. tubular basement membrane thickening, lamellation, and multilayering or presence of microcytes on EM examination. Genetic testing to confirm the diagnosis was not done due to financial constraints.
The other 8.1% had histopathological findings suggestive of CKDu, such as non-specific glomerular sclerosis, chronic interstitial nephritis, interstitial fibrosis, or lymphocytic infiltration on LM, negative immunostaining and nonspecific advanced chronic changes in EM. This was comparable to (7.4%) that was reported by Foula et al.6 This percentage is more amenable than previously thought to decrease if genetic testing was involved as concluded in recent studies that found 10–40% of cases of CKDu could be due to undiagnosed genetic diseases15,18,19 or genetic predisposition to CKDu.20–22
All patients with findings suggestive of CKDu were inhabitants of rural areas, but only 47% reported working as farmers cultivating sugarcane, wheat, or vegetables. Except for two young patients, the other nine patients were middle-aged, close to the age reported in India and Siri Lanka, with male sex predominance.2,11 None of them were diabetic, nor had a positive family history of renal diseases, or extrarenal manifestations. They had higher serum creatinine levels than reported in the literature. This may be due to the insidious onset and slow progression of their disease.
Amyloidosis (other than light chain amyloidosis) was found in 15% of our patients, which exceeded the percentage reported by Zaza et al. (7%).13 This may reflect the prevalence of underrecognized amyloidosis among Egyptians, especially the ALECT2 phenotype.23 Moreover, unsuspected light chain cast nephropathy was detected in 22 patients (10.5%). However, few cases had a similar presentation in the published literature.24,25 This supports the importance of multiple myeloma screening in cases of unexplained renal injury.26 We found that 12.6% of patients had vasculitis despite negative ANCA serology. This highlights the role of renal biopsies and not relying on serological tests to rule out this organ, or even life-threatening disease.
Forty percent of our patients had potentially treatable diagnoses by directed therapy beyond cardiovascular and CKD-MBD optimization. This was in agreement with Zaza et al. who found that treatment strategies remained unchanged after biopsy in patients with CKD, and only symptomatic therapy was continued in 60% of patients.13 However, Kitterer D et al. reported that renal histology resulted in identifying diseases potentially responsive to treatment modification in 74% of cases.27 This discrepancy may be attributed to the fact that most of our patients presented with advanced stages, as well as the presence of diagnoses that do not have a definite standard of care treatment i.e., CKDu, some genetically inherited disorders, or LECT amyloidosis. Besides, treatment modifications were undergone after biopsy results such as discontinuation of immunosuppression for patients with secondary FSGS, searching for inflammatory conditions for patients with AA amyloidosis, and cessation of the offending factors in ATN cases.
ConclusionOur study underlines the importance of renal biopsy in patients with unexplained renal impairment. It has a fundamental role in disease characterization, prognostication, and treatment optimization even in the presence of the advanced nature of renal involvement. Moreover, transplant decisions such as the risk of recurrence of the original disease, the possibility of relative donation, and the need for simultaneous organ transplantation have a pertinent relation to renal biopsy findings. In patients with unexplained renal impairment, glomerular diseases were the most common histological diagnosis, and FSGS and amyloidosis were the most frequent glomerular pathologies. Severe GS and IF were significantly associated with inferior outcomes. Finally, a more integrated primary healthcare program is needed to expedite early diagnosis, timely management, and subsequently prevent progression to CKD.
Authors’ contributionsEffat A. Tony: This author helped study design, writing, and editing of the manuscript.
Rabab Radi: This author helped data collection and excel sheets preparation.
Wesam Ismail: This author helped conduct of the study, data collection and draft writing of the manuscript.
Mohamed Ismail Seddik: This author helped conduct of the study and data collection.
Radwa A. Ellisy: This author helped the design of the study, data collection and draft writing of the manuscript.
Essam M. Abdel Aziz: This author helped in the final writing and editing of the manuscript.
Ethical approvalAll procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee at which the studies were conducted (IRB approval number IRB17200010), and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
FundingThe authors have no sources of funding to declare for this manuscript.
Conflicts of InterestThe authors have declared that no conflict of interest exists.
The authors declare no conflicts of interest
Data sharing informationRaw data (de-identified) used in this clinical trial are available from the corresponding author. It will be available following publication upon reasonable request.
It has not been published in other journals in whole or part.
It was prospectively registered at www.clinicaltrial.gov number; NCT03586531.