A renal biopsy represents the gold standard in the diagnosis, prognosis and management of patients with chronic kidney disease and glomerulonephritis. Strain wave elastography (SE) is a developing technique to assess tissue elasticity. The aim of this study was to correlate between the strain index value of renal parenchyma and degree of renal fibrosis detected with renal biopsy.
MethodFor 68 patients who were referred for a kidney biopsy, SE test was performed. The Banff scoring system was utilized to classify the IFTA grading of kidney fibrosis that assigns a severity level of mild, moderate, or severe. Receiver operating characteristic curve (ROC) was utilized to correlate between the severity of renal fibrosis and the grade of renal elasticity determined by SE.
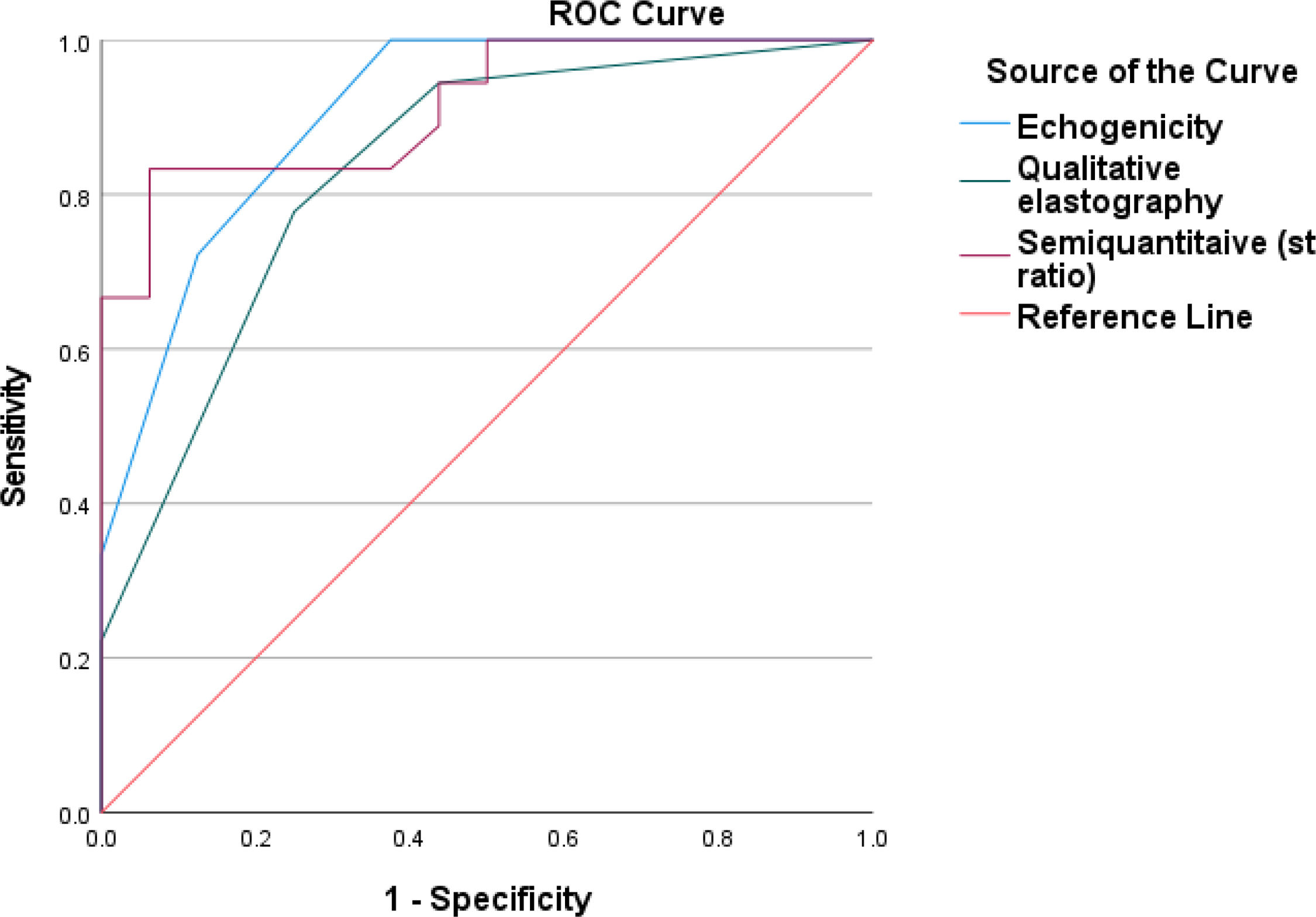
ResultsIn total, 38 males and 30 females, the echogenicity, qualitative and semiquantitative elastography showed significant positive correlation with serum creatinine, percentage of fibrosis, G score and tubular atrophy and significant negative correlation with eGFR. ROC curve of SE for diagnosis of interstitial fibrosis shown that echogenicity has sensitivity 100.0%, specificity 62.5%, positive predictive value (PPV) 75.0%, negative predictive value (NPV) 100.0% with area under curve (AUC) 0.906, while qualitative elastography has sensitivity 77.8%, specificity 75.0%, PPV 77.8%, NPV 75.0%, AUC 0.833, semi quantitative elastography has sensitivity 83.3%, specificity 93.8%, PPV 93.8%, NPV 83.3% with AUC 0.915.
ConclusionSE approach is simple to use, and can differentiate between varying stages of renal fibrosis. However, further research is required before it can be frequently used in clinical practice.
La biopsia renal constituye la técnica de referencia en términos de diagnóstico, pronóstico y manejo de los pacientes con enfermedad renal crónica y glomerulonefritis. La elastografía por ondas de corte (EOC) es una técnica en desarrollo para evaluar la elasticidad tisular. El objetivo de este estudio fue correlacionar el valor del índice de esfuerzo del parénquima renal y el grado de fibrosis renal, detectados mediante biopsia renal.
MétodoSe realizó EOC en 68 pacientes derivados para biopsia renal. Se utilizó el sistema de puntuación Banff para clasificar la IFTA de fibrosis renal que asigna un nivel de gravedad leve, moderado o severo. Se utilizó la curva ROC (receiver operating characteristic curve) para correlacionar la gravedad de la fibrosis renal y el grado de elasticidad renal determinado mediante EOC.
ResultadosEn un total de 38 varones y 30 mujeres, la ecogenicidad de la elastografía cualitativa y semicuantitativa reflejó una correlación positiva significativa con la creatinina sérica, el porcentaje de fibrosis, la puntuación G y la atrofia tubular, y una correlación negativa significativa con eGFR. La curva ROC de EOC para el diagnóstico de la fibrosis intersticial mostró que la ecogenicidad tuvo una sensibilidad (S) del 100%, una especificidad (E) del 62,5%, un valor predictivo positivo (VPP) del 75% y un valor predictivo negativo (VPN) del 100%, con un área bajo la curva (ABC) de 0,906, mientras que la elastografía cualitativa tuvo una S del 77,8%, una E del 75%, un VPP del 77,8% y un VPN del 75%, con un ABC de 0,833, y la elastografía semicuantitativa tuvo una S del 83,3%, una E del 93,8%, un VPP del 93,8% y un VPN del 83,3%, con un ABC de 0,915.
ConclusiónLa técnica EOC es simple de utilizar y puede diferenciar entre diversos tipos de fibrosis renal. Sin embargo, se necesita más investigación antes de utilizarse normalmente en la práctica clínica.
Chronic kidney disease (CKD) is emerging as a major global public health concern.1 Causes of CKD include primary renal diseases, diabetes mellitus and hypertension. As CKD worsens, there is extensive tissue scarring that eventually causes the kidney parenchyma to be destroyed. There is no way to reverse the pathologic damage, which can result in end stage renal disease (ESRD).2 Kidney biopsy is the gold standard when evaluating interstitial fibrosis, tubular atrophy and glomerulosclerosis. However, this invasive procedure may result in serious complications including bleeding.3 An alternative non-invasive method for evaluating pathological alterations is ultrasound elastography. There are various techniques for elastography including strain wave elastography (SE), transient elastography, shear wave elastography (SWE), and acoustic radiation force impulse (ARFI) imaging.4 In strain electrography, a transducer produces color images by measuring a displacement caused by pressure applied to the kidney. The images’ various hues correspond to varying degrees of stiffness.5 Because external pressure compression is necessary for SE to be completed, renal allografts were the subject of studies on SE due to their closer proximity to the body's surface.6 Shear wave elastography (SWE) is an additional ultrasound elastography method that uses ultrasound generated shear wave velocity (SWV), which functions as a virtual “finger” to detect tissue stiffness. In SWE, a shear wave that propagates perpendicular to the push-pulse is produced by tissue deformation brought on by an acoustic radiation force impulse (ARFI) or mechanical vibration caused by an ultrasonic instrument. It was discovered that ARFI was a potentially useful and promising technique for evaluating renal fibrosis and CKD. (The square of the SWV directly relates to tissue stiffness. SWE can evaluate renal stiffness in native kidneys as well as renal allografts without the need for external pressure compression.7 Various SWE-based techniques are currently accessible, such as point shear wave elastography (pSWE), transient elastography (TE), and 2D-SWE.8 A new economical, and noninvasive technique for assessing tissue elasticity is acoustic radiation force impulse (ARFI) imaging. When used in conjunction with ultrasound technology, ARFI imaging can yield both qualitative and quantitative evaluations of the parenchymal elasticity. In order to determine the mechanical characteristics of soft tissues in the region of interest (ROI), acoustic radiation force impulse imaging transiently deforms those tissues. The dynamic displacement response of those tissues is then monitored ultrasonically.9 Compared to other image-based elastography techniques, strain wave elastography was first used in clinical settings. Several researches have evaluated the feasibility of ultrasound elastography in CKD patients.10 However, most of the previous reports on the kidney were based on SWE and the results regarding the relationship between the SE and renal function or the CKD stage are not widely evaluated. Additionally, the role of SE in evaluation of renal fibrosis is not widely investigated in native kidneys, especially in correlation to results of renal biopsy. The purpose of the current study is to assess the correlation between strain index (SI) values of the renal parenchyma in patients and different stages of fibrosis evaluated by renal biopsy.
Patients and methodThis prospective cross-sectional study included 68 patients recruited from Internal Medicine Department, Nephrology unit, indicated for renal biopsy from May 2023 to April 2024. We included patients older than 18 years presented with proteinuria more than 1g, unexplained renal impairment or isolated glomerular hematuria. Patients with uncontrolled hypertension, solitary kidney, anatomical malposition, polycystic kidneys, thin renal parenchyma, active urinary or intraabdominal infections, renal malignancy or bleeding disorders were excluded from the study. Before biopsy, patients were advised to stop anticoagulant and antiplatelet for appropriate duration. All patients subjected to comprehensive history taking and thorough clinical examination. The laboratory investigations include complete urine analysis, 24-h urinary protein, blood urea, serum creatinine, serum albumin, total cholesterol (TC), triglycerides (TG), complete blood count (CBC), anti-nuclear antibodies (ANA), AntidsDNA, Antineutrophil Cytoplasmic Antibodies (ANCA) P&C, hepatitis C virus (HCV) antibodies, hepatitis B surface (HBs) antigen, C3, C4 and rheumatoid factor. The estimated glomerular filtration rate (eGFR) was calculated by The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) 2021equation.11
Kidney biopsy and histopathological examinationFollowing acquisition of the patients’ written informed permission, the kidney biopsies were obtained by the same nephrologists from the lower pole of the left kidney, using ultrasound guidance. All patients were kept for 24h in the hospital under strict monitoring to exclude out any complications.
All renal biopsies were examined by the same experienced nephropathologist. Hematoxylin and eosin (H&E), periodic acid-Schiff (PAS) and Masson trichrome stains were applied to slides containing 1–2μm thin slices from renal biopsies that were formalin-fixed and paraffin embedded. The slides were examined for diagnosis. The percentage of tubular atrophy, interstitial fibrosis, (IFTA) and global glomerular sclerosis were scored. Additionally, the examination of vascular sclerosis was carried out. The Banff scoring system was utilized to classify the IFTA grading of kidney fibrosis. This approach assigns a severity level of mild (fibrotic area<25%), moderate (26–50%), or severe (>50%).12
Stain wave elastography examinationPrior to elastography, all patients underwent a US examination to rule out perirenal hematomas. The strain wave elastography was done for all patients by the same radiologist within 3 days of renal biopsy. Ultrasonography, color Doppler ultrasonography (CDUS), and ultrasonography–elastography examinations were conducted using a Toshiba (Toshiba Medical Systems Corporation, Otawara, Japan) Aplio 500 ultrasound apparatus and a 3.5–5.0MHz convex probe. Following CDUS and greyscale B-mode, strain wave elastography; a software within ultrasonography machine was enabled. It is a semi-static and semi-quantitative approach. The tissue is compressed and decompressed by the operator. The contraction or expansion of the tissue in the direction of the compression is referred to as the “strain.” The lesion exhibited distortion and displacement as a result of the compression. Based on displacement, the software determines the lesion's elasticity score. Wave-like effects are produced by the compression and decompression stages. Using a free-hand method, 7–12 gentle repetitive compressions were made to create elastography images. Sinusoidal waves are the result of repeated compressions. The ultrasonography monitor shows the wave and the renal tissue. Three windows split apart on the monitor. Greyscale ultrasonography is shown in the first image, color-coded ultrasonography and elastography is shown in the second, and sinusoidal wave compression and decompression is shown in the bottom window. The various tissue stiffnesses are quantified and graphically depicted using a color scale. Firm areas are depicted in green with an intermediate consistency, soft portions in red, and hard areas in blue. The strain index (SI) serves as the technique's data unit that automatically calculated by the software and the measurement ought to be carried out during the decompression stage from the kidney's axial axis.13 To lessen the impact of anisotropy, the region of interest (ROI) was oriented so that its main axis ran as parallel to the main axis of the pyramids as feasible. Two ROIs were employed in the same depth. One was positioned on the renal sinus (reference ROI), and the other on the renal parenchyma. For statistical analysis, the mean of three measurements of the SI values from both the renal parenchyma and sinus were employed.14
Fig. 1 showing radiologist performing stain wave elastography examination
Statistical analysisThe collected data were statistically assessed using the Statistical Package for Social Studies, version 25 (IBM, Illinois, Chicago, USA). The distribution of the data was determined using the Shapiro–Wilk test. The numerical variables, which were reported as mean and standard deviations or median and interquartile range (IQR), were compared using the ANOVA (F) test in case of normally distributed quantitative variables or Kruskal–Wallis test in case of non-normally distributed quantitative variables. The quantity and percentage for the categorical variable were ascertained and compared using Chi-squared and Monte Carlo exact tests. Spearman Correlation Test (rs): was used to study the relationship (direction and power) between nonparametric variables. Correlation considered weak when it was from 0.0 to less than 0.25, moderate from 0.25 to less than 0.75 and strong from 0.75 to 1.0. Binary logistic regression was done for 2D US (B-mode) and SE as predictors of interstitial fibrosis (Mild vs. Moderate to marked). Receiver Operating Characteristic (ROC) curve was used for measuring the accuracy, sensitivity & specificity of 2D US (B-mode) and SE for diagnosis of interstitial fibrosis (Mild vs. Moderate to marked). Areas under the curve represents the accuracy, it ranges from a zero up to one (100%).
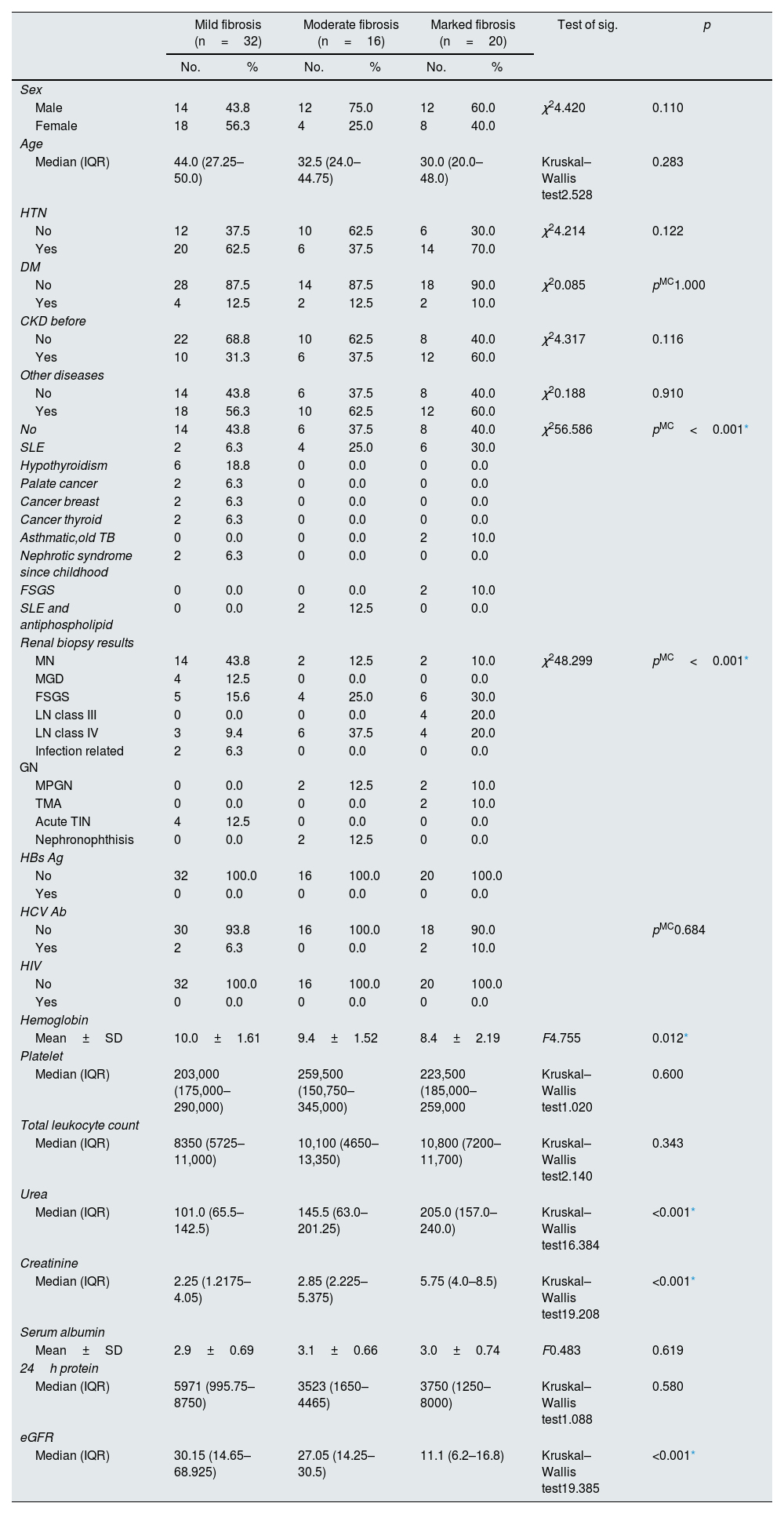
ResultsThe present study included 68 patients who had an indication for renal biopsy. The patients were divided according to degree of interstitial fibrosis into 3 groups. Group 1 included 32 patients with mild fibrosis, 14 males (43.8%) and 18 females (56.3%) with mean age 40.0±12.28 years. Group 2 included 16 patients with moderate fibrosis 12 males (75%) and 4 females (25%) with mean age 35.6±13.93 years. Group 3 included 20 patients with marked fibrosis, 12 males (60%) and 8 females (40%) with mean age 35.8±18.02 years. There were no statistically significant differences between the 3 groups regarding sex and age (P value 0.110 & 0.283) respectively (Table 1).
Demographic and laboratory data of the patients.
| Mild fibrosis (n=32) | Moderate fibrosis (n=16) | Marked fibrosis (n=20) | Test of sig. | p | ||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |||
| Sex | ||||||||
| Male | 14 | 43.8 | 12 | 75.0 | 12 | 60.0 | χ24.420 | 0.110 |
| Female | 18 | 56.3 | 4 | 25.0 | 8 | 40.0 | ||
| Age | ||||||||
| Median (IQR) | 44.0 (27.25–50.0) | 32.5 (24.0–44.75) | 30.0 (20.0–48.0) | Kruskal–Wallis test2.528 | 0.283 | |||
| HTN | ||||||||
| No | 12 | 37.5 | 10 | 62.5 | 6 | 30.0 | χ24.214 | 0.122 |
| Yes | 20 | 62.5 | 6 | 37.5 | 14 | 70.0 | ||
| DM | ||||||||
| No | 28 | 87.5 | 14 | 87.5 | 18 | 90.0 | χ20.085 | pMC1.000 |
| Yes | 4 | 12.5 | 2 | 12.5 | 2 | 10.0 | ||
| CKD before | ||||||||
| No | 22 | 68.8 | 10 | 62.5 | 8 | 40.0 | χ24.317 | 0.116 |
| Yes | 10 | 31.3 | 6 | 37.5 | 12 | 60.0 | ||
| Other diseases | ||||||||
| No | 14 | 43.8 | 6 | 37.5 | 8 | 40.0 | χ20.188 | 0.910 |
| Yes | 18 | 56.3 | 10 | 62.5 | 12 | 60.0 | ||
| No | 14 | 43.8 | 6 | 37.5 | 8 | 40.0 | χ256.586 | pMC<0.001* |
| SLE | 2 | 6.3 | 4 | 25.0 | 6 | 30.0 | ||
| Hypothyroidism | 6 | 18.8 | 0 | 0.0 | 0 | 0.0 | ||
| Palate cancer | 2 | 6.3 | 0 | 0.0 | 0 | 0.0 | ||
| Cancer breast | 2 | 6.3 | 0 | 0.0 | 0 | 0.0 | ||
| Cancer thyroid | 2 | 6.3 | 0 | 0.0 | 0 | 0.0 | ||
| Asthmatic,old TB | 0 | 0.0 | 0 | 0.0 | 2 | 10.0 | ||
| Nephrotic syndrome since childhood | 2 | 6.3 | 0 | 0.0 | 0 | 0.0 | ||
| FSGS | 0 | 0.0 | 0 | 0.0 | 2 | 10.0 | ||
| SLE and antiphospholipid | 0 | 0.0 | 2 | 12.5 | 0 | 0.0 | ||
| Renal biopsy results | ||||||||
| MN | 14 | 43.8 | 2 | 12.5 | 2 | 10.0 | χ248.299 | pMC<0.001* |
| MGD | 4 | 12.5 | 0 | 0.0 | 0 | 0.0 | ||
| FSGS | 5 | 15.6 | 4 | 25.0 | 6 | 30.0 | ||
| LN class III | 0 | 0.0 | 0 | 0.0 | 4 | 20.0 | ||
| LN class IV | 3 | 9.4 | 6 | 37.5 | 4 | 20.0 | ||
| Infection related GN | 2 | 6.3 | 0 | 0.0 | 0 | 0.0 | ||
| MPGN | 0 | 0.0 | 2 | 12.5 | 2 | 10.0 | ||
| TMA | 0 | 0.0 | 0 | 0.0 | 2 | 10.0 | ||
| Acute TIN | 4 | 12.5 | 0 | 0.0 | 0 | 0.0 | ||
| Nephronophthisis | 0 | 0.0 | 2 | 12.5 | 0 | 0.0 | ||
| HBs Ag | ||||||||
| No | 32 | 100.0 | 16 | 100.0 | 20 | 100.0 | ||
| Yes | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||
| HCV Ab | ||||||||
| No | 30 | 93.8 | 16 | 100.0 | 18 | 90.0 | pMC0.684 | |
| Yes | 2 | 6.3 | 0 | 0.0 | 2 | 10.0 | ||
| HIV | ||||||||
| No | 32 | 100.0 | 16 | 100.0 | 20 | 100.0 | ||
| Yes | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||
| Hemoglobin | ||||||||
| Mean±SD | 10.0±1.61 | 9.4±1.52 | 8.4±2.19 | F4.755 | 0.012* | |||
| Platelet | ||||||||
| Median (IQR) | 203,000 (175,000–290,000) | 259,500 (150,750–345,000) | 223,500 (185,000–259,000 | Kruskal–Wallis test1.020 | 0.600 | |||
| Total leukocyte count | ||||||||
| Median (IQR) | 8350 (5725–11,000) | 10,100 (4650–13,350) | 10,800 (7200–11,700) | Kruskal–Wallis test2.140 | 0.343 | |||
| Urea | ||||||||
| Median (IQR) | 101.0 (65.5–142.5) | 145.5 (63.0–201.25) | 205.0 (157.0–240.0) | Kruskal–Wallis test16.384 | <0.001* | |||
| Creatinine | ||||||||
| Median (IQR) | 2.25 (1.2175–4.05) | 2.85 (2.225–5.375) | 5.75 (4.0–8.5) | Kruskal–Wallis test19.208 | <0.001* | |||
| Serum albumin | ||||||||
| Mean±SD | 2.9±0.69 | 3.1±0.66 | 3.0±0.74 | F0.483 | 0.619 | |||
| 24h protein | ||||||||
| Median (IQR) | 5971 (995.75–8750) | 3523 (1650–4465) | 3750 (1250–8000) | Kruskal–Wallis test1.088 | 0.580 | |||
| eGFR | ||||||||
| Median (IQR) | 30.15 (14.65–68.925) | 27.05 (14.25–30.5) | 11.1 (6.2–16.8) | Kruskal–Wallis test19.385 | <0.001* | |||
χ2: Chi square test IQR (interquartile range) F: ANOVA.
Table 1 summarizes the demographic, clinical laboratory and histopathology parameters of the studied groups. There was statistically significant difference in the hemoglobin (Hb) level between the 3 groups (P value 0.012). However, there was no statistically significant difference between the 3 groups considering platelet count and total leukocyte count (P value 0.600 & 0.343 respectively). There was statistically significant difference between the 3 groups regarding blood urea and serum creatinine (P value<0.001 & <0.001 respectively). The eGFR had statistically significantly difference between the 3 groups (P value<0.001*) with highest median eGFR was present in group 1 while the lowest values were present in group 3. Additionally, there was non- statistically significant difference between the 3 groups regarding serum albumin level (P value 0.619) and 24-h urinary protein (P value 0.580) which is explained by the fact that most of the patients in group 1 have MGN.
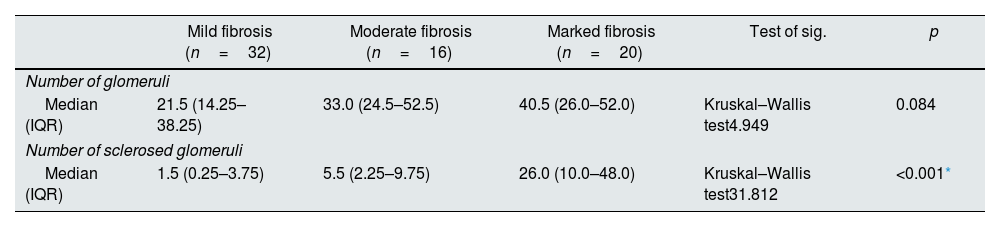
The statistical analysis of the histopathological examination demonstrated that the number of glomeruli in renal biopsy core had no statistically significant difference among the 3 groups (P value 0.084). However, the number of sclerosed glomeruli had statistically significant difference (P value<0.001*) with the largest number of sclerosed glomeruli presented in group 3. The degree of tubular atrophy, the percentage of interstitial fibrosis and G score showed statistically significant difference between studied groups (P value <0.001*, <0.001* & <0.001*) respectively. On the other hand, there was no statistically significant difference between groups as regard presence of crescent (P value 0.685) (Table 2).
Histopathological and elastography findings of the patients.
| Mild fibrosis (n=32) | Moderate fibrosis (n=16) | Marked fibrosis (n=20) | Test of sig. | p | |
|---|---|---|---|---|---|
| Number of glomeruli | |||||
| Median (IQR) | 21.5 (14.25–38.25) | 33.0 (24.5–52.5) | 40.5 (26.0–52.0) | Kruskal–Wallis test4.949 | 0.084 |
| Number of sclerosed glomeruli | |||||
| Median (IQR) | 1.5 (0.25–3.75) | 5.5 (2.25–9.75) | 26.0 (10.0–48.0) | Kruskal–Wallis test31.812 | <0.001* |
| No. | % | No. | % | No. | % | |||
|---|---|---|---|---|---|---|---|---|
| Tubular atrophy | ||||||||
| No | 20 | 62.5 | 6 | 37.5 | 2 | 10.0 | χ274.339 | pMC<0.001* |
| Mild atrophy | 12 | 37.5 | 2 | 12.5 | 0 | 0.0 | ||
| Moderate atrophy | 0 | 0.0 | 8 | 50.0 | 2 | 10.0 | ||
| Marked atrophy | 0 | 0.0 | 0 | 0.0 | 16 | 80.0 | ||
| % of fibrosis | ||||||||
| Mean±SD. | 15.0±5.39 | 36.9±7.27 | 64.5±10.11 | F270.230 | <0.001* | |||
| Min.–Max. | 5.0–25.0 | 25.0–45.0 | 55.0–90.0 | |||||
| G score | ||||||||
| G0 | 8 | 25.0 | 0 | 0.0 | 0 | 0.0 | χ247.547 | pMC<0.001* |
| G1 | 18 | 56.3 | 12 | 75.0 | 2 | 10.0 | ||
| G2 | 6 | 18.8 | 4 | 25.0 | 6 | 30.0 | ||
| G3 | 0 | 0.0 | 0 | 0.0 | 12 | 60.0 | ||
| Crescent | ||||||||
| No | 26 | 81.3 | 14 | 87.5 | 18 | 90.0 | χ20.832 | pMC0.685 |
| Yes | 6 | 18.8 | 2 | 12.5 | 2 | 10.0 | ||
| Elastography findings of the patients | ||||||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |||
| Echogenicity | ||||||||
| Normal | 20 | 62.5 | 0 | 0.0 | 0 | 0.00 | χ239.856 | pMC<0.001* |
| Mild increased | 8 | 25.0 | 6 | 37.5 | 4 | 20.0 | ||
| Moderate increased | 4 | 12.5 | 6 | 37.5 | 8 | 40.0 | ||
| Marked increased | 0 | 0.0 | 4 | 25.0 | 8 | 40.0 | ||
| Qualitative elastography | ||||||||
| Red/green scale | 18 | 56.3 | 2 | 12.5 | 0 | 0.0 | χ229.932 | pMC<0.001* |
| Green scale | 6 | 18.8 | 4 | 25.0 | 2 | 10.0 | ||
| Blue/green scale | 8 | 25.0 | 8 | 50.0 | 12 | 60.0 | ||
| Blue scale | 0 | 0.0 | 2 | 12.5 | 6 | 30.0 | ||
| Semiquantitative (SI) | ||||||||
| Mean±SD | 1.1±0.50 | 2.6±1.62 | 2.8±0.90 | Kruskal–Wallis test35.820 | <0.001* | |||
The statistical analysis of the elastography findings revealed that there were statistically significant differences between the studied groups regarding echogenicity, qualitative and semi quantitative (SI) elastography between studied groups (P value<0.001*, <0.001* & <0.001* respectively) (Table 2, Figs. 2–4).
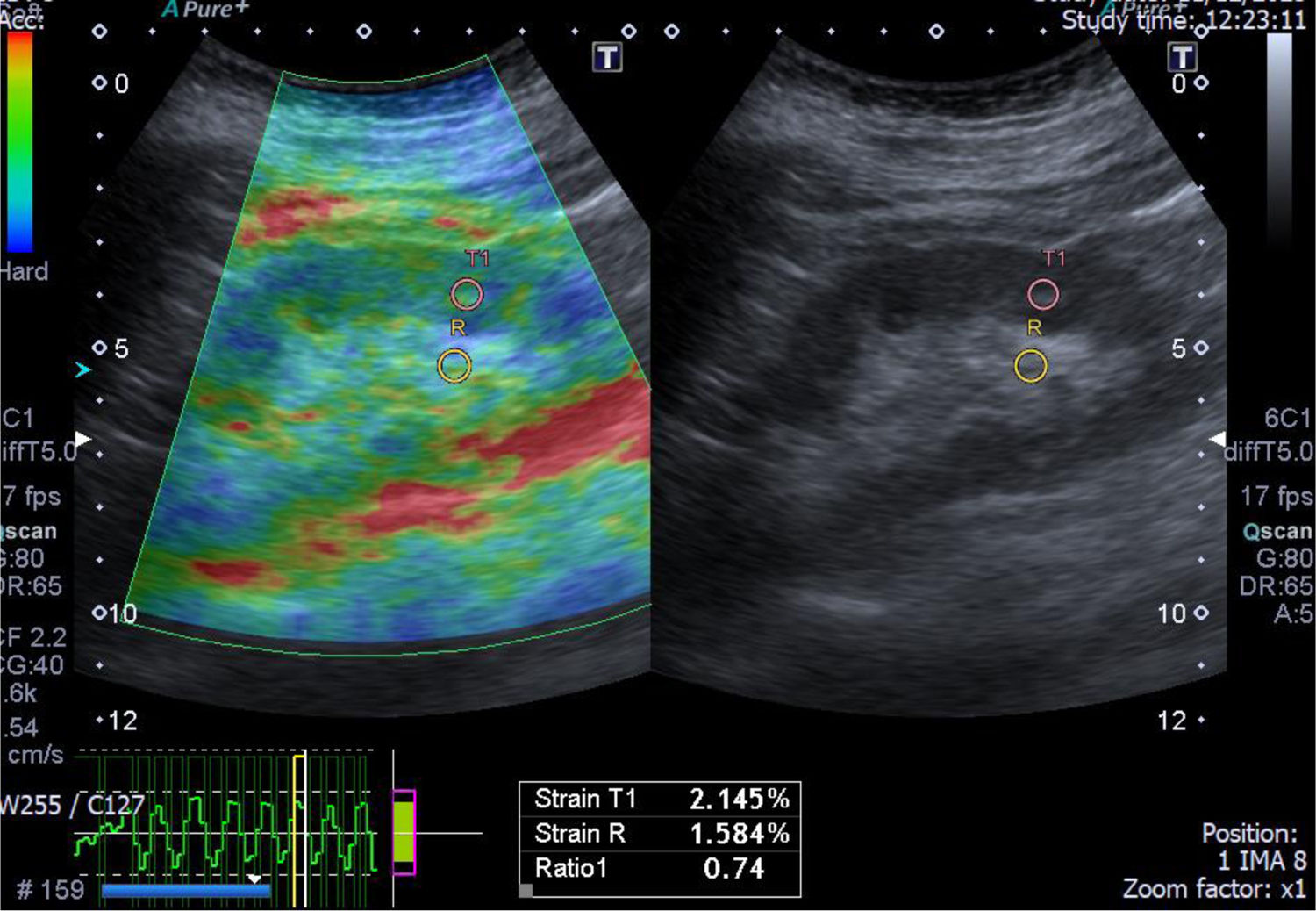
A case of acute interstitial nephritis post chemotherapy with mild interstitial fibrosis (10%). The greyscale ultrasonography image showing mild increased parenchymal echogenicity with preserved cortico-medullary differentiation, the left one showing color-coded US – elastography image showing mainly green-red scale and the sinusoidal wave of compression and decompression seen in inferior aspect of image. The circles indicate the region of interests (ROIs). The upper ROI is on the parenchyma and the lower ROI is on renal fat sinus. The radial line on the sinusoidal wave indicates the end measurement (SI=0.74).
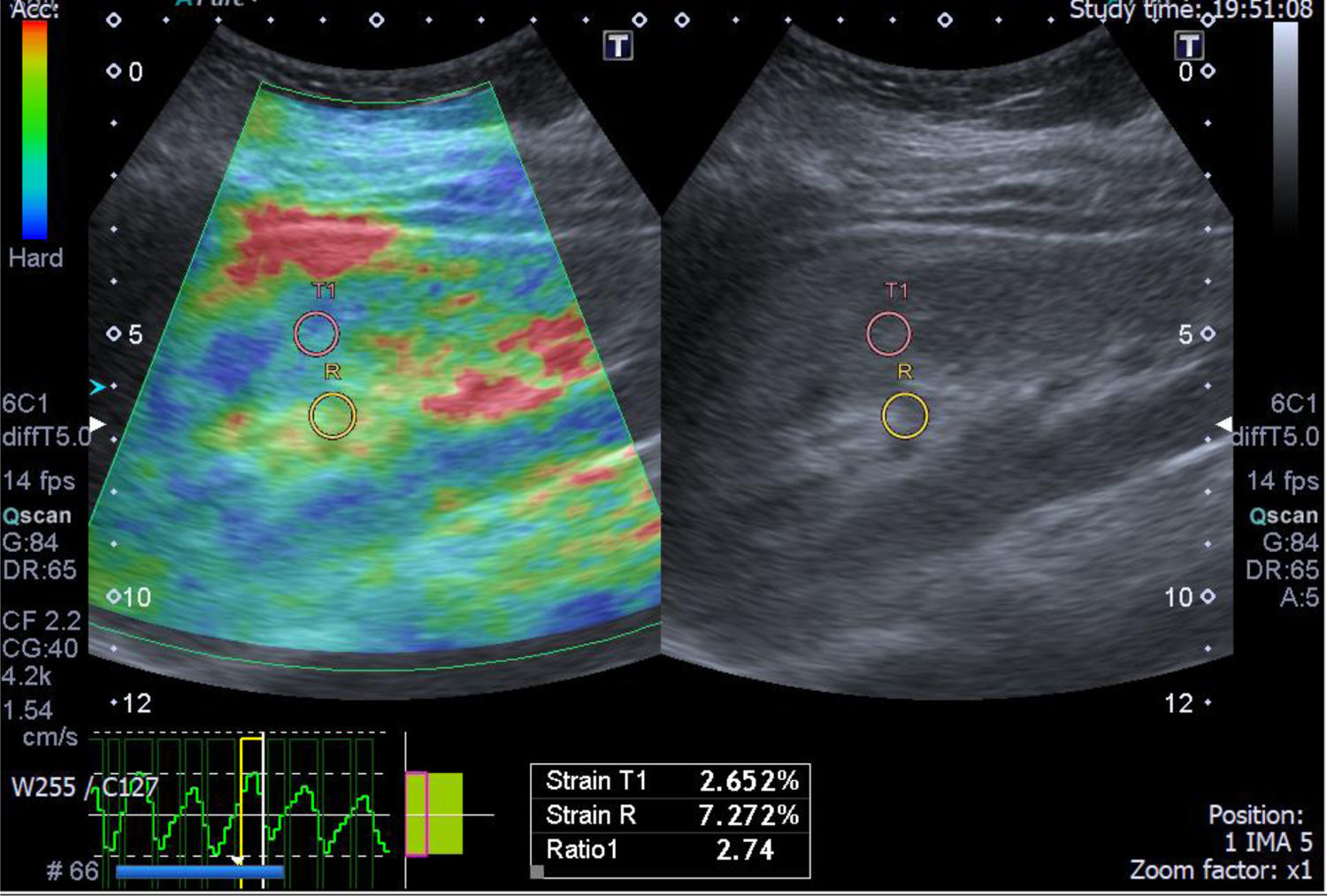
A case of light chain renal amyloidosis with moderate interstitial fibrosis (25%). The greyscale ultrasonography image showing moderate increased parenchymal echogenicity with preserved cortico-medullary differentiation, the left one showing color-coded US – elastography image showing mixed green-blue scale and the sinusoidal wave of compression and decompression seen in inferior aspect of image. The circles indicate the region of interests (ROIs). The upper ROI is on the parenchyma and the lower ROI is on renal fat sinus. The radial line on the sinusoidal wave indicates the end measurement (SI=2.74).
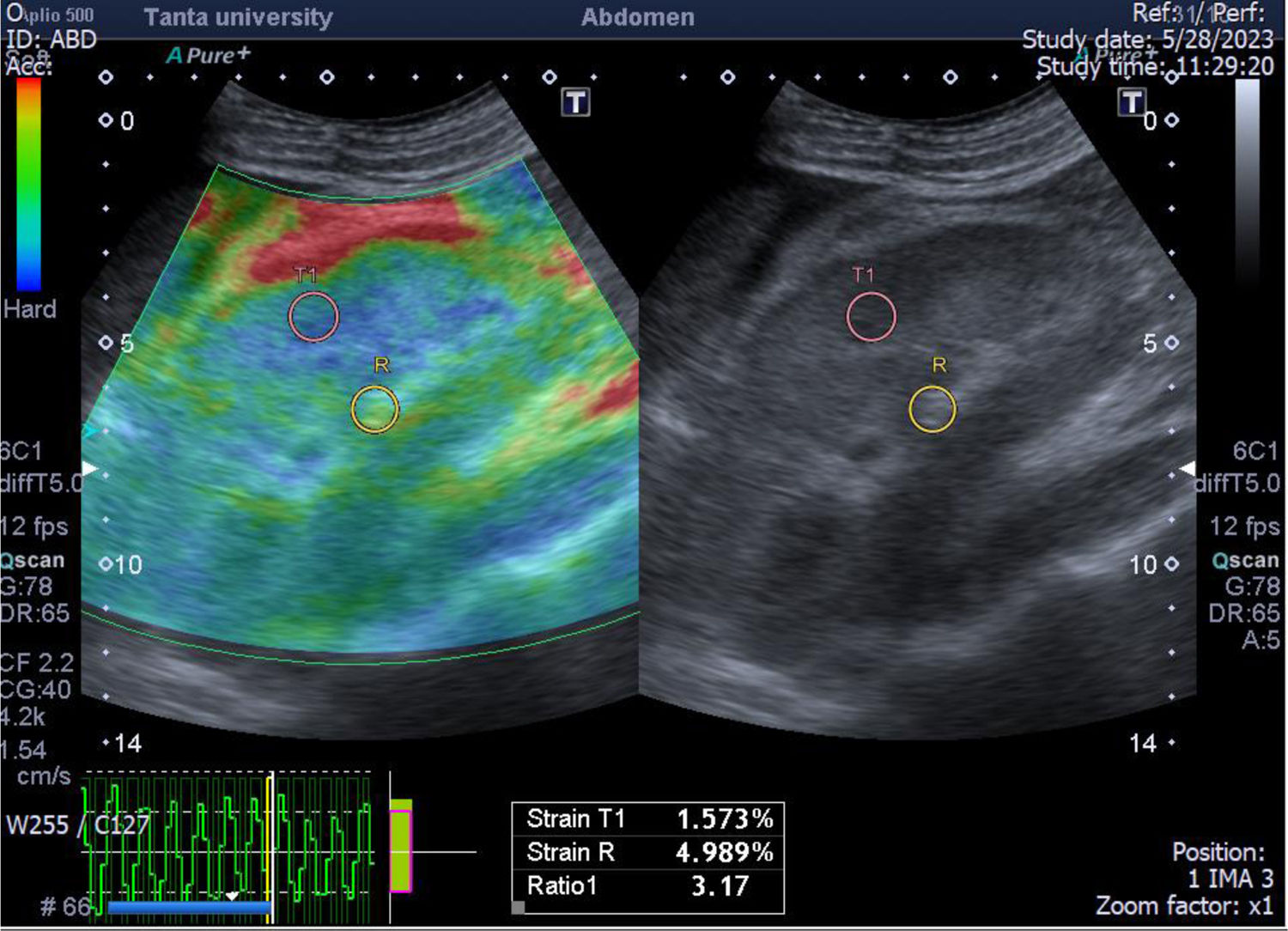
A case of focal necrotizing GN with small vessel vasculitis with marked interstitial fibrosis (55%). The greyscale ultrasonography image showing marked increased parenchymal echogenicity with relatively poor cortico-medullary differentiation, the left one showing color-coded US – elastography image showing mainly blue-green scale and the sinusoidal wave of compression and decompression seen in inferior aspect of image. The circles indicate the region of interests (ROIs). The upper ROI is on the parenchyma and the lower ROI is on renal fat sinus. The radial line on the sinusoidal wave indicates the end measurement (SI=3.17).
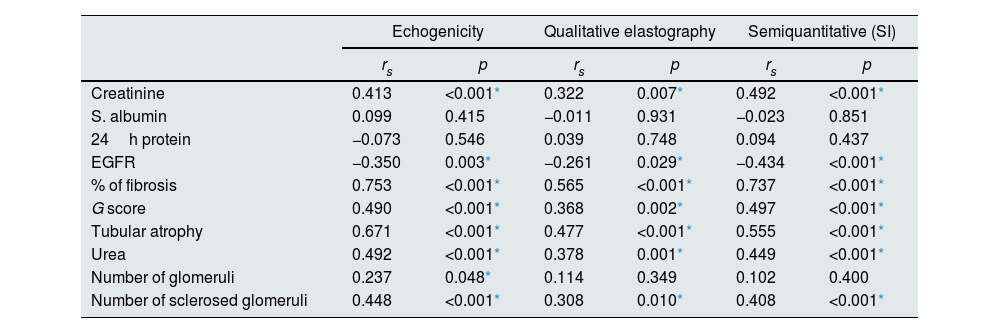
Spearmen correlation showed that the echogenicity, qualitative and semiquantitative elastography (SI) showed significant positive correlation with blood urea, serum creatinine, percentage % of fibrosis, G score, degree of tubular atrophy and number of sclerosed glomeruli and significant negative correlation with eGFR (Table 3).
Spearman correlation between elastography & other variables.
| Echogenicity | Qualitative elastography | Semiquantitative (SI) | ||||
|---|---|---|---|---|---|---|
| rs | p | rs | p | rs | p | |
| Creatinine | 0.413 | <0.001* | 0.322 | 0.007* | 0.492 | <0.001* |
| S. albumin | 0.099 | 0.415 | −0.011 | 0.931 | −0.023 | 0.851 |
| 24h protein | −0.073 | 0.546 | 0.039 | 0.748 | 0.094 | 0.437 |
| EGFR | −0.350 | 0.003* | −0.261 | 0.029* | −0.434 | <0.001* |
| % of fibrosis | 0.753 | <0.001* | 0.565 | <0.001* | 0.737 | <0.001* |
| G score | 0.490 | <0.001* | 0.368 | 0.002* | 0.497 | <0.001* |
| Tubular atrophy | 0.671 | <0.001* | 0.477 | <0.001* | 0.555 | <0.001* |
| Urea | 0.492 | <0.001* | 0.378 | 0.001* | 0.449 | <0.001* |
| Number of glomeruli | 0.237 | 0.048* | 0.114 | 0.349 | 0.102 | 0.400 |
| Number of sclerosed glomeruli | 0.448 | <0.001* | 0.308 | 0.010* | 0.408 | <0.001* |
ROC curve of SE for diagnosis of interstitial fibrosis (Mild vs. Moderate to marked) has shown that echogenicity has sensitivity 100.0%, specificity 62.5%, positive predictive value (PPV) 75.0%, negative predictive value (NPV) 100.0% with area under curve (AUC) 0.906 while qualitative elastography has sensitivity 77.8%, specificity 75.0%, PPV 77.8%, NPV 75.0% AUC 0.833. Semi quantitative elastography (SI) has sensitivity 83.3%, Specificity 93.8%, PPV 93.8%, NPV 83.3% with AUC 0.915 (Fig. 5).
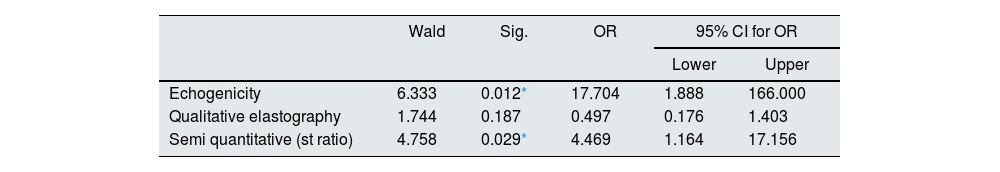
As predictors of interstitial fibrosis, binary logistic regression for SE showed that echogenicity and semi quantitative elastography (SI) had statistically significant values (Mild vs. Moderate to marked) (P value 0.012* & 0.029* respectively). However, qualitative elastography had statistically non-significant values (P value 0.187) (Table 4).
Binary logistic regression for two-dimensional US (B- mode) and Strain wave elastography as predictors of interstitial fibrosis (Mild vs. Moderate to marked).
Globally, ESRD is becoming more common.15 Renal fibrosis and scarring are common in CKD patients, and both conditions can eventually result in kidney failure. As such, early renal fibrosis diagnosis and surveillance are necessary for improving the prognosis and management of CKD with various etiologies.16 Due to the paucity of research on the most effective noninvasive markers, kidney biopsy is still the gold standard for fibrosis and etiology identification despite its invasive nature and related risks.17 In general, regardless of the tissue or organ, fibrosis tends to increase tissue stiffness.18 Elastography is primarily used to evaluate the renal stiffness consequently, forecast renal fibrosis.19 Because of the kidney's diverse parenchymal setting, elastography is more challenging than in the liver.20 SE was widely investigated in renal allograft due to their closer proximity to the body's surface.21 Renal ultrasound elastography has been evaluated in transplanted kidneys, renal malignant tumors and in patients with various kidney diseases. The majority of earlier studies on the kidney were based on SWE.22
Numerous investigations have examined the applicability of using ultrasound elastography to evaluate renal neoplasms.22 The renal elasticities of 19 individuals with renal cell carcinomas and 28 patients with angiomyolipomas were examined by Tan et al. According to their findings, strain elastography could be used to distinguish renal angiomyolipomas from renal cell carcinomas by analyzing the elasticity patterns.23 Göya and colleagues examined the capacity to distinguish benign from malignant kidney cancers. They demonstrated how the SWVs in infectious lesions, malignant tumors, and benign lesions differed from those in normal parenchyma. Among them, the nearby renal parenchyma's SWV was considerably higher than the hematoma's. Compared to benign lesions, the SWVs in malignant tumors were noticeably higher. On the other hand, the SWVs of malignant and infectious tumors did not significantly differ from one another.24 on the other hand, the elasticity of renal malignant tumors in relation to lesion size was studied by Cai et al. Patients with solid renal tumors less than 4cm in size and malignancy were recruited. The elasticity values of the malignant masses, which were primarily made up of clear cell carcinomas, were found to be lower than those of the benign angiomyolipomas. They proposed that the SWE values were affected by the heterogeneity inside the tumor.25
The value of ultrasonography elastography in kidney transplant recipients has been the subject of numerous investigations. The connection between the pathological alterations and elasticity, specifically in interstitial fibrosis, is debatable.22 The relationship between renal elasticity and the Banff score or interstitial fibrosis was examined by Stock et al. through the use of SWE in 18 renal transplants. The SWV, the degree of fibrosis, and the Banff score were found to positively correlate with one another.26 on the other hand, the association between the grade of fibrosis and the SWV in kidney transplant recipients was examined by Syversveen et al. Regarding the degree of fibrosis, the SWVs in 30 renal transplant patients did not exhibit any significant differences.27 Different histological alterations and times following transplantation may account for the differences in these reports.22
The present study was conducted as to predict kidney fibrosis in patients with renal diseases using two-dimensional ultrasound (B-mode) and strain elastography images in combination with renal biopsy. The results of the present study showed that echogenicity and semi quantitative elastography (SI) were significant predictors for fibrosis (P value 0.012* & 0.029* respectively). However, qualitative elastography had statistically non-significant values (P value 0.187).
In agreement with our results, S. Menzilcioglu et al. compared the renal parenchyma between 58 CKD patients and 40 healthy individuals by SE. The mean SI showed statistically significant difference between normal individuals (0.42±0.30) and CKD patients regardless of stages (1.81±0.88) (P 0.001). However, with the exception of CKD Stages 1 and 3, SI values were not statistically significant across all CKD stages. For SI, the ROC area under the curve was 0.956. The best cut-off value for CKD prediction was 0.935, which had an 88% sensitivity and a 95% specificity. They concluded that elastography's SI value can be used to distinguish between healthy people and CKD patients, however it hadn’t been demonstrated to be able to accurately distinguish between different stages.10
Considering that fibrosis causes a rise in tissue stiffness, Guo et al. evaluated the renal parenchymal stiffness in 64 CKD patients and 327 healthy individuals with acoustic radiation force impulse (ARFI) elastography technique. Although they used quite different method, they showed similar results and the outcome is unaffected by the elastography method. They found a significant difference in shear wave velocity (SWV) between the CKD patients and control group. Additionally, they created a cut-off value for SWV of 1.88m s21 with 69.7% specificity and 71.9% sensitivity using the ROC curve. However, ARFI elastography approach was unable to distinguish between different phases of CKD.28 Moreover, despite using different method of elastography, Ayu Makita et al. correlated between renal elasticity by real time elastography and the extent of fibrosis in 29 patients underwent renal biopsy. They showed that renal elasticity of native kidneys was significantly positively correlated with the grade of renal fibrosis (P=0.003). At the cutoff point of 3.81, the area under the curve, sensitivity, and specificity were 0.778, 68.4%, and 81.8%, respectively. In agreement to our results, they concluded that Real-time tissue elastography is a promising, non-invasive method for assessing renal fibrosis in patients with CKD.29
Hassan et al. assessed the degree of renal fibrosis in 29 patients with diabetic kidney disease (DKD) and 23 healthy participants by SWE in patients with DKD (especially stage 4 CKD) the cortical stiffness was higher than healthy subjects (P<0.001), In agreement to our results, they showed significant negative correlation between cortical stiffness and eGFR (r=−0.65, P<0.001). The 24-h proteinuria correlated positively with cortical stiffness (r=0.56, P<0.001). Although the present study showed positive correlation between 24-h proteinuria and renal fibrosis, it was not statistically significant.30
The limitations of this study include a small sample size and the short duration of the study that did not allow to follow up the renal outcome correlated to fibrosis staging. Additionally, we used SE which presumed to assess the strain in deeply situated organs as a native kidney; however, it is undeniable that the depth from the skin may have an impact on how the tissue strain is captured.
ConclusionStrain wave elastography may be an alternative non-invasive technique in assessing and follow up the extent of renal fibrosis in a native kidney. However, renal biopsy remains the gold standard for diagnosis etiology of CKD.
Informed consentInformed consent was obtained from all individual participants included in the study.
Ethical approvalThe study was approved by the Research Ethics Committee at the Faculty of Medicine, Tanta University (Approval code: 36264PR224/6/23).
FundingNo fund was available for the research.
Conflict of interestThe authors declared no conflict of interest.
Availability of data and materialsThe datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.