Renal ischemia-reperfusion occurs in some clinical conditions such as kidney surgery that can leads to acute renal failure. The aim of this study was to investigate the effect of p-coumaric acid (CA) on ischemia reperfusion (I/R) injury.
MethodsThirty rats were randomly divided into five groups; control, CA (100mg/kg), I/R, propylene glycol (10%)+I/R and CA+I/R, (n=6 each). CA and propylene glycol were administered orally for 2 weeks. Then, the rats were subjected to bilateral renal ischemia for 45min and followed by reperfusion for 24h. All rats were killed and kidney function tests, tissue malondialdehyde and activity of antioxidant enzymes were determined. Histopathological evaluations were also performed. In addition, renal expression of the tumor necrosis factor-α and interleukin-1β were determined using enzyme-linked immunosorbent assay and immunohistochemistry.
ResultsCA significantly improved the Cr and BUN levels in CA+I/R group compared to I/R group (p<0.005 and p<0.001, respectively). Reduction of tissue superoxide dismutase, glutathione peroxidase and catalase, were significantly improved by CA (p<0.01, p<0.01 and p<0.05). Treatment with CA also resulted in significant reduction in tissue MDA (p<0.05), TNF-α (p<0.001) and interleukin-1β expression (p<0.001) that were increased by renal I/R. Also, the rats treated with CA had nearly normal structure of the kidney.
ConclusionsThe present findings suggest that, CA protects the kidneys against I/R injury via its antioxidant and anti-inflammatory effects.
La isquemia-reperfusión renal se produce en algunas situaciones clínicas como la cirugía renal, y puede conducir a insuficiencia renal aguda. El objetivo de este estudio fue investigar el efecto del ácido p-cumárico (AC) en el daño por isquemia-reperfusión (I/R).
MétodosSe dividió aleatoriamente a 30 ratas en 5 grupos; control, AC (100mg/kg), I/R, propilenglicol, (10%)+I/R y AC+I/R, (n=6 cada uno). El AC y el propilenglicol se administraron por vía oral durante 2 semanas. A continuación, las ratas se sometieron a isquemia renal bilateral durante 45min, seguido de reperfusión durante 24h. Se sacrificó a todas las ratas y se determinaron los valores de la función renal, el malondialdehído tisular y la actividad de las enzimas antioxidantes. También se llevaron a cabo evaluaciones histopatológicas. Además, se determinó la expresión renal del factor de necrosis tumoral-α y la interleucina-1β mediante enzimoinmunoanálisis de adsorción e inmunohistoquímica.
ResultadosEl AC mejoró significativamente los niveles de Cr y BUN en el grupo de AC+I/R en comparación con el grupo de I/R (p<0,005 y p<0,001, respectivamente). La reducción de la superóxido-dismutasa tisular, la glutatión-peroxidasa y la catalasa mejoró significativamente con el AC (p<0,01, p<0,01 y p <0,05, respectivamente). El tratamiento con AC también provocó una reducción significativa de la expresión del malondialdehído (MDA) tisular (p<0,05), el TNF-α (p<0,001) y la interleucina-1β (p<0,001) que habían aumentada por la I/R renal. Además, las ratas tratadas con AC presentaron una estructura renal casi normal.
ConclusionesEstos hallazgos sugieren que el AC protege los riñones frente al daño por I/R a través de sus efectos antioxidantes y antinflamatorios.
Renal ischemia-reperfusion (I/R) injury takes place in various clinical situations including intense hypotension and following resuscitation, transplantation of kidney, incomplete nephrectomy and cardiovascular operations that can all cause acute renal failure (ARF).1 It is demonstrated using a response of local inflammatory resulted from an primary ischemia and pursued by reperfusion, which causes formation of reactive oxygen species (ROS).2–4 ROS have different cytotoxic impacts, comprising damage of DNA, oxidation of protein and nitrosylation, lipid peroxidation, and apoptosis induction.5 Sign of oxygen radical-mediated injury in the kidney is renal injury demonstration being accentuated with oxidants and the observation that shortage of antioxidants exacerbates renal injury and that free radical-mediated lipid peroxidation occurs as a sign of IR injury also implicate oxidants in the pathophysiology of ARF.6 In addition, ischemic yields mediators including proinflammatory cytokines (e.g., TNF-α, IL-1β, TGF-β) and chemotactic cytokines like monocyte chemoattractant protein-1 (MCP-1) and IL-8.7 Renal IR injury is related with very high morbidity and mortality because restricted therapeutic modalities available.3 Thus, new and effective therapeutic approaches are necessary greatly. Recently, the biologically active materials obtained from herbals have been used for preventing and treating acute and chronic illnesses.8 Polyphenolic compounds are defined as chemopreventive agents. p-Coumaric acid (CA), a kind of polyphenolic compound, is hydroxy derivative of cinnamic acid that is observed in numerous edible herbs ingested by humans and animals.9,10 It is extensively available in a plenty of foods including grapes, red and white wine, spinach, tomato, carrot, garlic and coffee.11 CA has attracted considerable attention because of its numerous pharmacological and biological activities, including chemoprotective,12 neuroprotective,13 cardioprotective,14 antioxidant (radical scavenging),15 anti-microbial, anti-cancer, anti-ulcer activity.16 Keeping the above facts in view, this research assessed antioxidant properties of CA against kidney I/R injury in rats.
We analyzed the antioxidant enzymes (superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), malondialdehyde (MDA)) levels and various inflammatory mediators, such as tumor necrosis factor-α (TNF-α), interleukin-β (IL-1β) in rats with renal I/R which pre-treated with CA and vehicle.
Material and methodsMaterialsTNF-α ELISA kit was purchased from Diaclone (Besançon, France). SOD and GPx kits were from Randox Lab (Crumlin, UK). Antibody against IL-1β and horseradish peroxidase conjugated secondary antibody were from Zellbio GmbH (Germany). p-Coumaric acid (purity >98%) and other reagents were obtained from Sigma–Aldrich (St. Louis, Mo, USA).
Thirty Sprague-Dawley rats weighing from 190 to 210g were purchased from the animal house of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. The rats were kept in controlled temperature (22±2°C) with humidity 50–55% and under 12h light/12h dark cycle. During the period of experiment the animals were permitted free access to water and a conventional diet ad libitum.
Experimental protocolThe rats were randomly divided into 5 groups of 6 rats each.
Group I (control), animals received distilled water.
Group II (CA), animals received p-cumaric acid (100mg/kg/d).
Group III (I/R), animals were exposed to I/R.
Group IV (Vehicle+I/R), animals in this group were given propylene glycol 10% and were exposed to I/R.
Group V (CA+I/R), animals in this group were treated with p-cumaric acid (100mg/kg/d) followed by I/R.
CA was dissolved in 10% propylene glycol and also, all treatments were oral. Animals were pre-treated for 2 weeks and then were subjected to I/R.
Induction of renal ischemic reperfusion injuryAll groups except those in groups I and II were anesthetized intraperitoneally with ketamine (75mg/kg) and xylazine (10mg/kg), a midline incision was made, and both left and right renal pedicles were identified and occluded with two smooth atraumatic vascular clamps for 45min. After 45min, the vessel clamps were removed and the kidneys were checked for blood flow restoration. After unclamping of the renal pedicles, the abdominal wall was sutured and the animals were placed in individual metabolic cages. Animals in groups I and II endured a similar surgical procedure without renal pedicle occluding. After reperfusion for 24h, rats were anesthetized, blood and kidney samples were taken for more studies.
Measurement of biochemical parametersBlood samples were collected from right ventricular and then centrifuged with 6000×g for 3min. Concentrations of blood urea nitrogen (BUN) and serum creatinine (Cr) were measured spectrophotometrically using a commercially available kit (parsazmon kit, Iran).
Measurement of biomarkers of oxidative stressRight kidneys were cut into two halves. One half of kidneys weighed and homogenized 1:20 in 0.1M potassium phosphate buffer (pH 7.4) then, homogenates were centrifuged at 16,000×g for 20min at 4°C. The supernatants were used for measurement of biomarkers of oxidative stress include: MDA, SOD, CAT and GPx.
MDA level in renal tissue was determined by thiobarbituric acid reactivity as previously described.17 An aliquot (500μl) of the supernatant to formation of protein precipitate was added to 1500μl trichloroacetic acid (10%) and centrifuged at 4000×g for 10min and 1500μl of supernatant was mixed with 2ml thiobarbituric acid (0.67%). Then the mixture was boiled for 30min at 100°C. After cooling, centrifuged at 4000×g for 15min and the supernatant absorbency was read at 535nm by spectrophotometry. Values were expressed as μmol/mg protein, based on the standard curve prepared from standard solution of MDA.
SOD activity was measured using Ransod kit (Randox Labs, Crumlin, UK). This method uses xanthine oxidase and xanthine to produce superoxide radicals that react with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride (INT) to create a red formazan. One unit of SOD was described as the amount causing 50% inhibition in the INT reduction rate. SOD activity is expressed as units/mg protein.
GPx activity was measured using Ransel kit (Randox Labs, Crumlin, UK). GPx accelerates the oxidation of glutathione by cumene hydroperoxide. The oxidized glutathione in the presence of NADPH and glutathione reductase, changed to the diminished form associated with oxidation of NADPH to NADP+. The reduce in absorbance was determined at 340nm with a spectrophotometer. Activity is expressed as U/mg protein.
CAT activity was determined according to the method that described previously.18 Briefly, 2.9ml of H2O2 (19mM) in phosphate buffer (50mM, pH 7.0) was placed in a cuvette and the reaction started by adding supernatant (100μml). The changes of absorbance per min was recorded at 240nm. CAT activity was determined by using the extinction coefficient of 0.0436cm2μmol−1 H2O2. The CAT activity was calculated as U/mg protein.
Measurement of proteinMeasurement of protein concentration carried out by Bradford's method using bovine serum albumin as protein standard.19
Renal histological examinationLeft kidneys from rats of all the groups were fixed in 10% formaldehyde, dehydrated in graded ethanol and embedded in paraffin and fine (5μm) sections were prepared and mounted on glass slides. The sections were stained with PAS (periodic acid schiff) for light microscopy (Olympus, Tokyo, Japan) evaluation at 300× magnification. Histological injury was analyzed using a tubular injury score.20 Briefly, injury was scored in a blinded method based on the injury percentage included tubular dilatation, destroy of brush border, interstitial tissue hemorrhage and intratubular cast: 0, no injury; 1, <25%; 2, 25–50%; 3, 50–75%; 4, >75%. Histological damage was examined in 10 microscopic fields.
Measurement of TNF-α levelTNF-α concentrations were measured in the kidney tissue21 using enzyme-linked immunosorbent assay (ELISA) kit specific for rats according to the manufacturer's instructions. 100mg of renal tissue was homogenized in 1ml of PBS contain anti-protease cocktail for preventing of protein destruction. The samples were then centrifuged at 6000×g for approximately 10min and the supernatant used for ELISA assay.
Immunohistochemical analysis of IL-1βDetection of IL-1β in kidney sections was determined by immunohistochemistry as previously described.22 Briefly, sections were incubated overnight with primary antibody (rabbit anti-IL-1β at the dilution 1:400) at 4°C. Negative controls were incubated with normal serum instead of primary antibody. After washing with PBS sections were incubated with secondary conjugated antibody (goat anti-rabbit IgG/HRP conjugate) for 60min and then were washed with PBS. Finally, location of antigen-antibody complex was revealed by chromogen diaminobenzidine. The results of the immunohistochemical staining were categorized into 4 categories namely light (+), moderate (++), severe (+++) and very severe (++++). For each slide, 15 different microscopic fields were evaluated.
Statistical analysisData were expressed as mean±SEM. Study groups data were compared with one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison tests. P values <0.05 was considered to be significant.
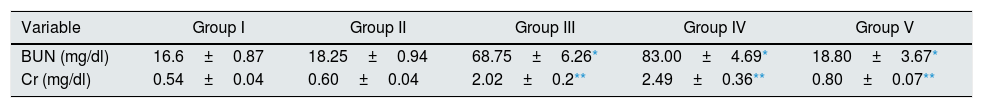
ResultsEffect of CA on renal functionAnimals in groups of I/R and Vehicle+I/R exhibited significant increase in the serum concentrations of Cr (p<0.005) and BUN (p<0.001) levels as compared to control animals. Pretreatment with CA (Group V) significantly improved the Cr and BUN levels (p<0.005) and (p<0.001). The Cr and BUN levels of CA group (Group II) were similar to control group (p>0.05) (Table 1).
Effect of p-coumaric acid (CA) pretreatment on serum Cr and BUN.
| Variable | Group I | Group II | Group III | Group IV | Group V |
|---|---|---|---|---|---|
| BUN (mg/dl) | 16.6±0.87 | 18.25±0.94 | 68.75±6.26* | 83.00±4.69* | 18.80±3.67* |
| Cr (mg/dl) | 0.54±0.04 | 0.60±0.04 | 2.02±0.2** | 2.49±0.36** | 0.80±0.07** |
The results are mean±SEM for six rats in each group. Group I (control), received distilled water; Group II (CA), received p-cumaric acid (100mg/kg/d for 2 weeks); in Group III (I/R), rats were exposed to ischemia for 45min and reperfusion for 24h; in Group IV (Vehicle+I/R), rats received propylene glycol 10% and were exposed to I/R and in Group V (CA+I/R), rats were treated with p-cumaric acid (100mg/kg/d) followed by I/R. Groups III and IV compared to control and Group V compared to Group III.
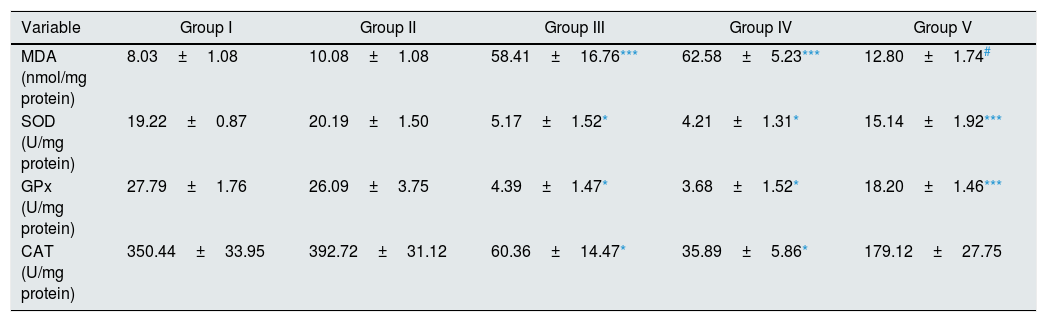
As illustrated in Table 2, there were statistically significant increase in renal tissue MDA level of I/R and Vehicle+I/R groups compared to control (p<0.01). Pretreatment with CA significantly improved MDA concentration (p<0.05). Also, the activities of antioxidant enzymes (SOD, GPx, CAT) decreased in groups of I/R and Vehicle+I/R compared to control group (p<0.001). Pretreatment with CA significantly increased antioxidant enzymes activity in Group V (p<0.01, p<0.01 and p<0.05). But, MDA concentration and antioxidant enzymes activity in CA group were similar to control group.
Effect of p-coumaric acid (CA) pretreatment on tissue oxidant and antioxidant enzyme activities.
| Variable | Group I | Group II | Group III | Group IV | Group V |
|---|---|---|---|---|---|
| MDA (nmol/mg protein) | 8.03±1.08 | 10.08±1.08 | 58.41±16.76*** | 62.58±5.23*** | 12.80±1.74# |
| SOD (U/mg protein) | 19.22±0.87 | 20.19±1.50 | 5.17±1.52* | 4.21±1.31* | 15.14±1.92*** |
| GPx (U/mg protein) | 27.79±1.76 | 26.09±3.75 | 4.39±1.47* | 3.68±1.52* | 18.20±1.46*** |
| CAT (U/mg protein) | 350.44±33.95 | 392.72±31.12 | 60.36±14.47* | 35.89±5.86* | 179.12±27.75 |
The results are mean±SEM for six rats in each group. Group I (control), received distilled water; Group II (CA), received p-cumaric acid (100mg/kg/d); in Group III (I/R), rats were exposed to I/R; in Group IV (Vehicle+I/R), rats received propylene glycol 10% and were exposed to I/R and in Group V (CA+I/R), rats were treated with p-cumaric acid (100mg/kg/d) followed by I/R. Groups III and IV compared to control and Group V compared to Group III.
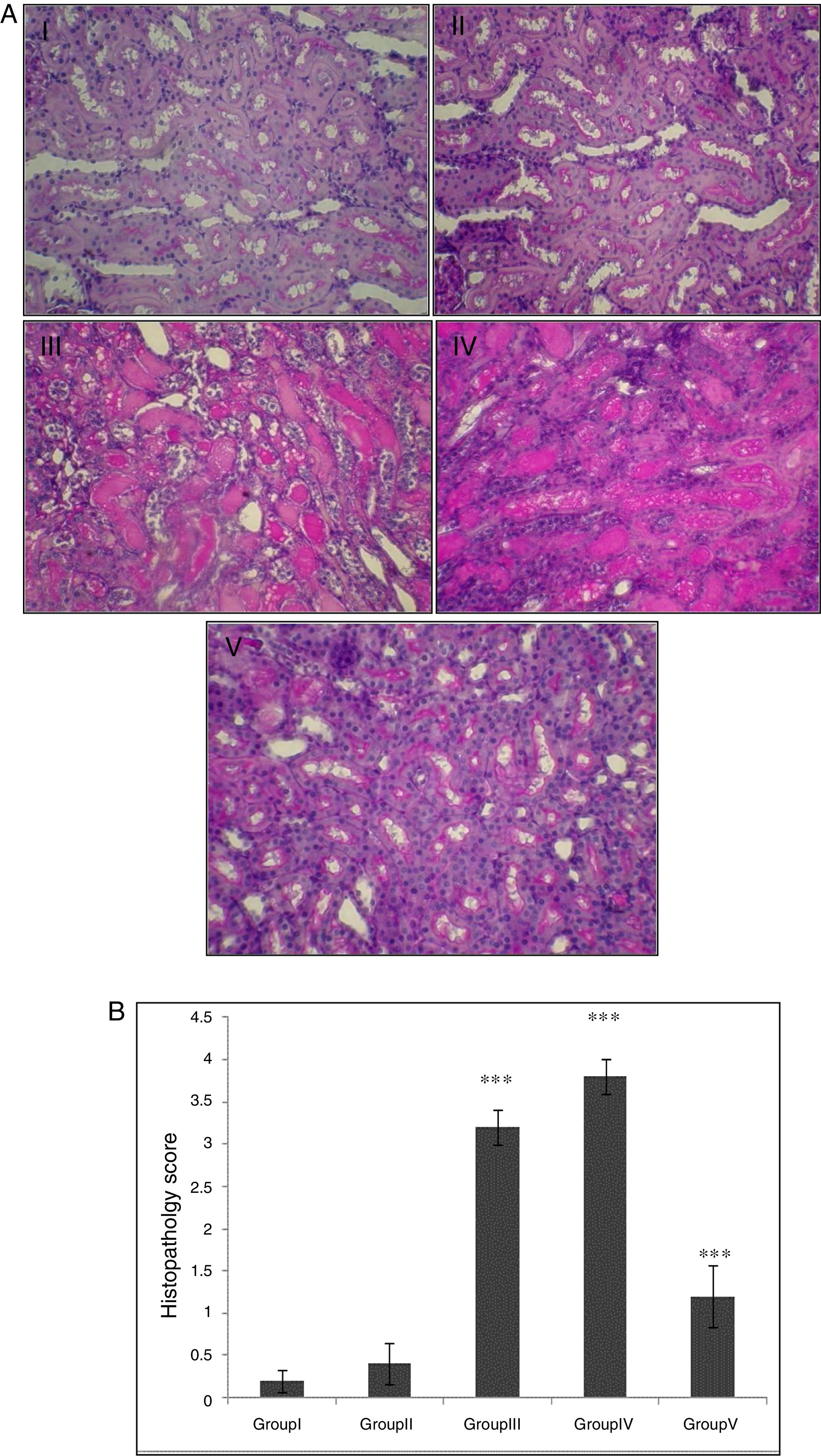
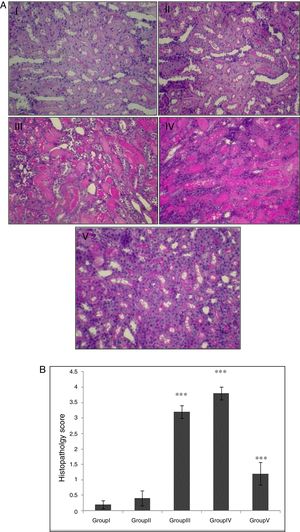
In the control group, renal tissue had a normal structure. No significant pathological changes were observed in the CA group. Tissue from the I/R and Vehicle+I/R groups revealed, tubular lumen dilation, vacuolization of tubule cells, degeneration of some of tubules and the accumulation of castes in the tubular lumen. Tissue sections from rats pretreated with CA showed marked decline of the structural features of renal damage (Fig. 1A). The histopathological changes were graded (Fig. 1B) and the kidneys of I/R and Vehicle+I/R rats had a score of 3.2±0.2 and 3.8±0.2 while treatment with CA nearly preserved normal structure of the kidney with a score of 1.2±0.37 (p<0.001).
(A) Light micrographs of the rats kidney sections (PAS). Group I (control), received distilled water; Group II (CA), received p-cumaric acid (100mg/kg/d); in Group III (I/R), rats were exposed to I/R; in Group IV (Vehicle+I/R), rats received propylene glycol 10% and were exposed to I/R for 45min and in Group V (CA+I/R), rats were treated with p-cumaric acid (100mg/kg/d for 2 weeks) followed by I/R. Kidney sections from control and p-coumaric acid (CA) groups with normal renal structure (I, II), I/R and Vehicle+I/R groups show severe renal injury such as; tubular lumen dilation, vacuolization of tubule cells, degeneration of some of tubules and The accumulation of castes in the tubular lumen (III, IV), the rats treated with CA prior to I/R process show relatively well-preserved structure (V). (B) Renal injury was scored. Groups III and IV compared to control and Group V compared to Group III. Values are shown as mean±SEM; *p<0.001 (n=6).
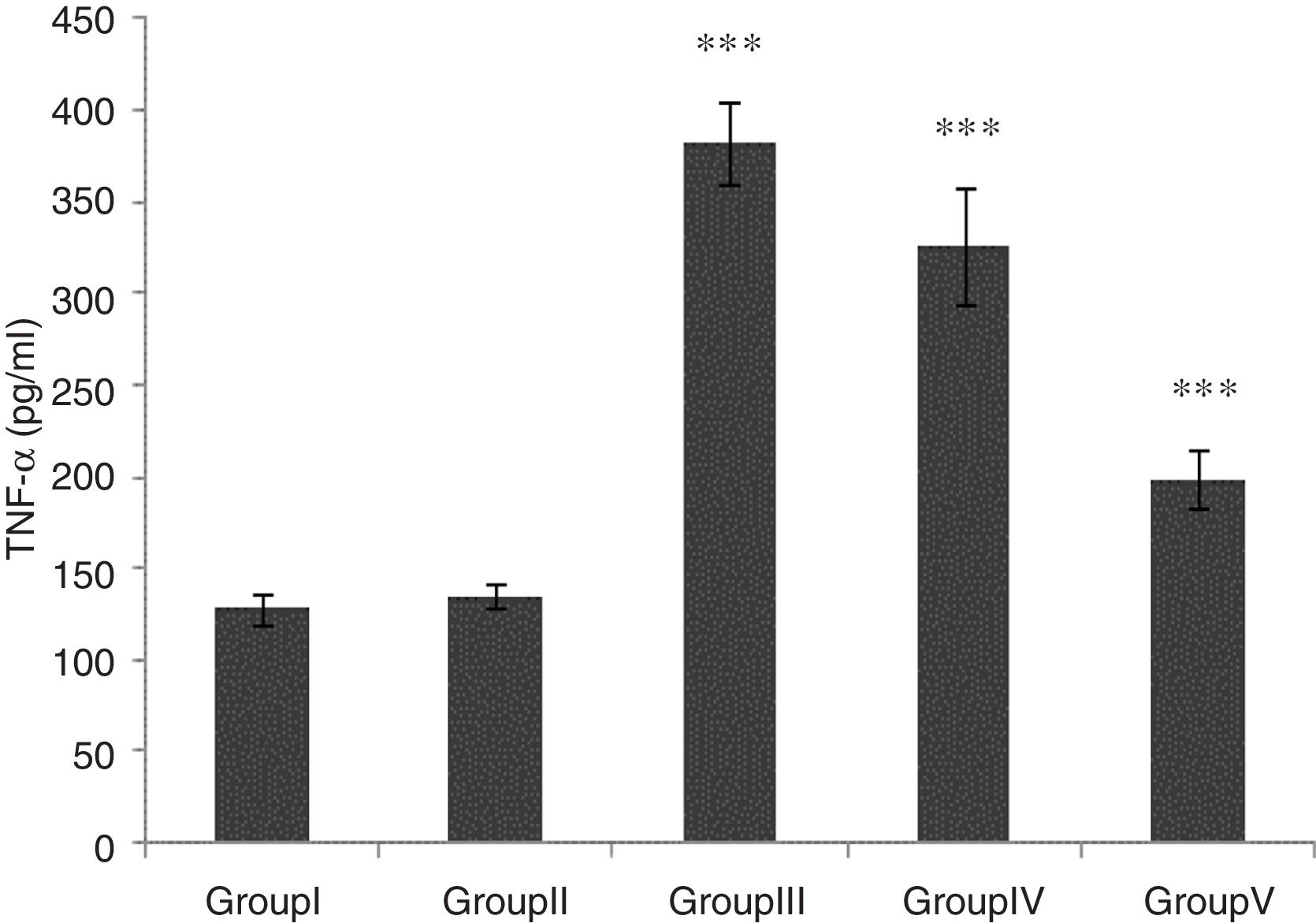
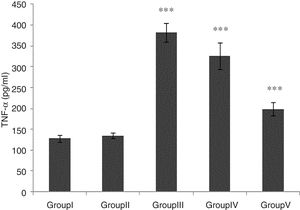
TNF-α level was significantly higher in groups of I/R and Vehicle+I/R compared with the control group (p<0.001). The TNF-α level were significantly lower in CA+I/R group than group I/R (p<0.001), however there was no significant difference between groups of control and CA. (Fig. 2).
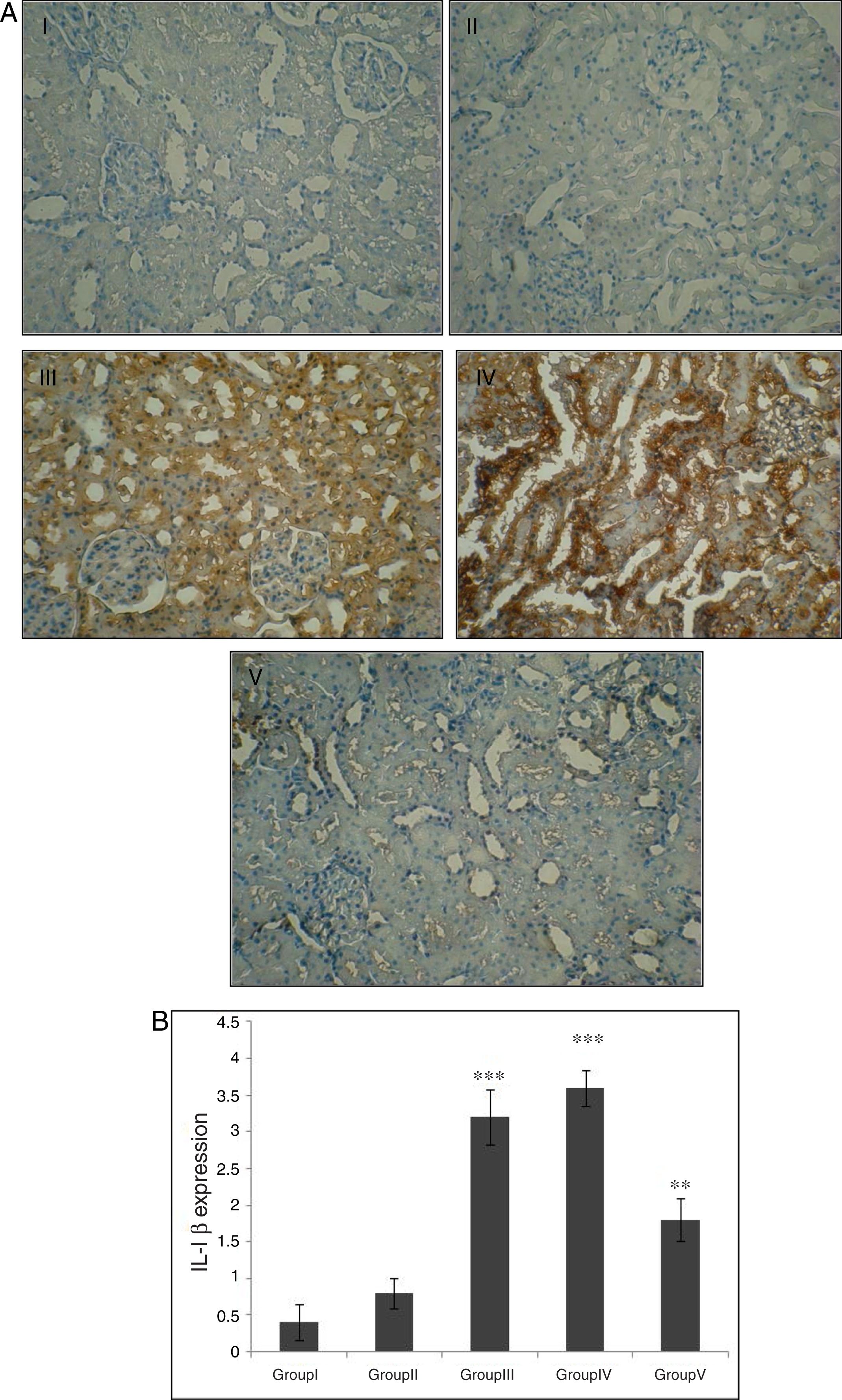
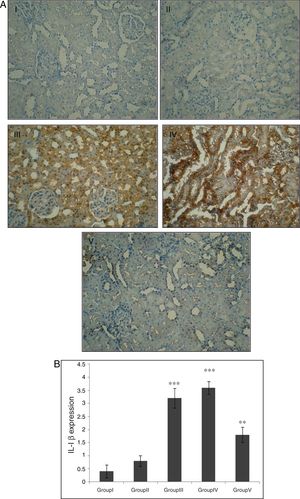
Effect of CA on IL-1β expression in kidney tissueStaining for IL-1β by immunohistochemistry demonstrated that the expression of IL-1β was increased after IR in groups of III and IV when compared to control group (p<0.001). A marked reduction in the staining for IL-1β was observed in kidneys obtained from CA+I/R group when compared with kidneys from I/R group (p<0.005). There was no significant difference in IL-1β expression between groups of control and CA (Fig. 3A). Immunoreactivity degrees of IL-1β is summarized in Fig. 3B.
Effect of CA pretreatment on IL-1β expression in kidney. (A) Immunohistochemistry for IL-1β demonstrated that the expression of IL-1β was increased after IR in groups of ІІІ and IV when compared to control group (p<0.001). A marked reduction in the IL-1β expression was observed in kidney tissues of CA+I/R group when compared with kidneys from I/R group. (B) Expression degree of IL-1β. Groups III and IV compared to control and Group V compared to Group III. Values are shown as mean±SEM; *p<0.001, **p<0.0005 (n=6).
The outcomes of our research indicate that pretreatment with p-cumaric acid decreases the subsequent I/R injury in the kidney, as indicated by ameliorated renal function, normalized renal histopathology, development in status of antioxidant enzyme (enhanced levels of SOD and activity of GPx, CAT) and decreased oxidation yields (decreased MDA level). The pathophysiology after renal I/R injury is not well recognized. The mechanisms are most probably multifactorial and interdependent containing hypoxia, inflammatory reactions, and damage of free radical.23 In recent years, the role of oxidative stress in the I/R injury has been studied widely. MDA is a sign of oxidative stress and also a main product of lipid peroxidation. SOD is an index of anti-oxidative capacity, involving in reversing the pathological variations in oxidative damage. It is well known that SOD, CAT, and GPx have key roles in the internal defense system versus oxygen free radicals.24,25 In general, enhanced MDA and reduced SOD, CAT, GPx in kidney tissue following IR has been recognized.26,27 In the current research, administration of p-cumaric acid prior IR reduced renal MDA and enhanced antioxidant enzymes of endogenous. These outcomes show that CA may decrease the formation of ROS and enhance ROS removal for normalizing the imbalance between the oxidative status and anti-oxidative after IR. Similar outcomes were achieved in previous research.28
The ARF was shown with enhanced Cr and BUN. These factors are signs of glomerular filtration rate.29 In this research, serum Cr and BUN levels in I/R rats were considerably more than those in control rats. This showed that renal dysfunction happened after I/R operation. Our outcomes presented that pre-treatment of CA decreased the increase of BUN and Cr induced through operation of ischemia reperfusion. This indicated that pre-treatment of CA was useful in preventing renal dysfunction induced by IR. In our research, the kidney of rats that experienced I/R indicated characteristic morphological variations such as brush border loss, sloughing of tubular epithelium from the basal lamina, and formation of intratubular cast. Renal sections achieved from rats pretreated with CA revealed noticeable decrease of the histological properties of renal injury. CA may successfully protect the tubular epithelium from reperfusion damage. The similar outcomes were observed by using other antioxidants.30,31
Inflammation has been identified as the main driving force in the ischemic procedure, and growing evidence has revealed that elevated levels of inflammatory signs are associated to ischemia.32,33 Tissue I/R injury causes activation of cascades, which upregulate cytokines such as; TNF-α, IL-1 β, TGF-β and plays a key role in the beginning of systemic inflammatory response. A research reported that accumulation of neutrophil and the level of cytokines containing TNF-α and IL-1β were increased in the reperfused kidney.34,35 Moreover, other research reported that the tissue expression of TNF-α and IL-1β was enhanced after renal I/R.36 The inflammatory cytokines including TNF-α and IL-1β are small secreted proteins that mediate and control inflammation. The inflammatory stresses induced through IR were reflected through elevation of TNF-α and IL-1β. Increasing evidence reports which TGF-β1 inhibits of matrix metalloproteinases expression in monocytes and macrophages induced by cytokines, such as IL-1β and TNF-α. Also, TGF-β increased in recovering of Kidney tubules from renal IR. This increased expression of TGF-β and its receptors during recovery after IRI, suggests the function of an augmented autocrine signaling loop.37 Studies have indicated which ischemia-reperfusion induces an increase in latent TGF-β levels but a decrease in its active form. Autoinduction of TGF-β expression as well as supplementation with exogenous TGF-β can protect organs from ischemia-reperfusion injury.38 However, the role of TGF-β was not examined in this study due to some limitations.
CA reduced amount of TNF-α and IL-1β in the kidney tissue, which proposed that their renoprotective properties were also related with anti-inflammatory features.
ConclusionsFrom this investigation, we could verify that p-coumaric acid protects the kidney against ischemia-reperfusion damage by reducing Cr, BUN, IL-1β, TNF-α and MDA levels, increasing antioxidative enzyme activities. The findings of the current study illustrate that p-coumaric acid, with its potent free radical scavenging, antioxidant and anti-inflammatory properties, seems to be a highly promising agent in protecting renal tissue against oxidative damage and in preventing renal dysfunction due to ischemia/reperfusion.
Ethical approvalAll procedures performed in this study were approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (IR.AJUMS.REC.1396.259). Animal care and handling was done in accordance with National Institutes of Health guidelines.
Conflict of interestThe authors have declared that no conflict of interest exists.
This paper was extracted from MSc student thesis (Shahin Mozaffari) that supported by the Vice Chancellor of Research Affairs of Ahvaz Jundishapur University of Medical Sciences (Grant no. CMRC-9612), Ahvaz, Iran.