The bone and mineral disorders form an integral part of the management of a chronic kidney disease (CKD) patient. Amongst various types of bone pathologies in chronic kidney disease-mineral bone disorder (CKD-MBD), the prevalence of adynamic bone disease (ABD) is increasing. The present review discusses the updated pathophysiology, risk factors, and management of this disorder.
Los trastornos óseos y minerales son una parte fundamental del tratamiento del paciente con enfermedad renal crónica (ERC). Entre los distintos tipos de patologías óseas en la enfermedad renal crónica-trastorno mineral óseo (ERC-TMO), la prevalencia de la enfermedad ósea adinámica (EOA) está aumentando. En esta revisión se analizan los datos actuales sobre la fisiopatología, los factores de riesgo y el tratamiento de este trastorno.
The term adynamic bone disease (ABD) or aplastic bone disease was first described in early 1980s.1,2 ABD is defined by faulty rate of collagen synthesis and mineralization i.e. low-bone turnover without osteoid accumulation (thin osteoid seam). It needs to be differentiated from osteomalacia which is also a low turnover disease but their defect in mineralization exceeds the defects in bone formation and therefore, there is a relative osteoid excess in osteomalacia. In ABD, there are few or no osteoblasts, minimal or no peri-trabecular fibrosis, substantially low bone formation rate (BFR) and the number of re-modeling sites is also low.3,4 Here, we would be briefly summarizing ABD in the following sections with special focus on newer treatment strategies.
ABD in course of CKD and prevalenceABD is most common of all CKD associated MB disorders (osteitis fibrosa, osteomalacia, mixed turnover disorder), and requires preventive care even from early stages of CKD. Though, advances CKD stages are at high risk of ABD with prevalence rate variable from 10 to 40% in advanced stages to 10–50% in dialysis patients but it is also not uncommon to see in earlier CKD stages 3–5 with a prevalence rate showed rising trends and reaches to about 18%.5,6 In one study of patients with pre-dialysis CKD, ABD was seen in 12% of bone biopsies. Increased average age, percentage of patient with diabetes and high vitamin D and oral calcium supplements are important contributor to the rising trends.7 Peritoneal dialysis (PD) is more commonly associated with ABD (almost in 50%) than hemodialysis (HD).8
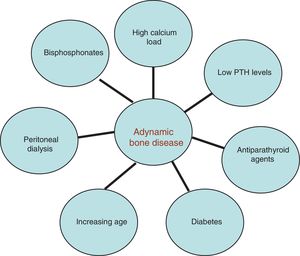
Risk factorsThe risk factors for ABD are multifactorial (Fig. 1). Elderly individuals, diabetes, high calcium load, vitamin D excess, overzealous treatment of hyperparathyroidism, low parathyroid hormone (PTH) levels, parathyroidectomy, systemic inflammation, calcimimetics, and bisphosphonates have all been commonly implicated.9,10
High concentrations of glucose and insulin deficiency suppresses PTH secretion in parathyroid cell cultures.11 In a study of rats with streptozocin-induced diabetes, bone histology was characterised by low bone formation, reduced osteoid measurements, decreased osteoclast number, and less bone collagen synthesis.12
High calcium load, whether oral dietary intake or in dialysis solution and low phosphate diet suppresses parathyroid gland hyperplasia by induction of p21 and reduction of transforming growth factor alpha. In addition, calcitriol also induces p21 and act via membrane trafficking of the epithelial growth factor receptor (EGFR) and down regulated signaling.13 Adynamic bone is thus a result of over-suppression of PTH synthesis and secretion.14
CKD progression is coupled with PTH resistance due to changes in the tissue expression of their regulators, disturbances of hepatic and renal PTH catabolism and downregulation or desensitization of PTH1R.15 It is also affected by serum level of phosphate, indoxyl sulphate and paracresyl sulphate. In addition, treatment resulting excess of CaSR and vitamin D receptor activation along with FGF23-Klotho endocrine axis suppresses PTH secretion. FGF23 binds to the Klotho to activate MAPK-pathway and there is also an early inhibition of the Wnt pathway with an increase in the expression of sclerostin.16,17
Bisphosphonates are risk factor in advanced CKD for ABD in presence of abnormal laboratory parameters. Metanalysis of bisphosphonates in age related CKD 1–3 concluded it as a safe therapy in absence of laboratory features of CKD-MBD.18 In another study of 31 CKD stage 5 hemodialysis patients, short term usage of bisphosphonates did no harm and bone biopsy was not advised.19 However, study of thirteen CKD patients (stage 2–4) treated from 4 to >60 months with bisphosphonates for osteopenia or osteoporosis, underwent trabecular bone biopsies from the iliac crest and all were diagnosed with adynamic bone on biopsy evaluation.20 It is therefore necessary to warn for a possible harm before administering bisphosphonates in this patient population. KDIGO suggests that treatment choices including bisphosphonates take into account the magnitude and reversibility of the biochemical abnormalities and the progression of CKD, with consideration of a bone biopsy in patients with CKD G3a-G5D with biochemical abnormalities of CKD-MBD and low BMD and/or fragility fractures.21
Other potential mechanisms of low bone formation include elevated circulating cytokine levels (interleukin (IL)-I, tumor necrosis factor (TNF), low estrogen and testosterone levels, decreased osteoblast proliferation from the direct effect of accumulated uremic inhibitors, and decreased circulating insulin-like growth factor (IGF)-I activity either from low IGF-I and/or IGF-binding protein (IGFBP)-5 levels or from excess IGFBPs that inhibit the action of IGF-I.22–29
ABD pathophysiologyThree elements of bone characterize different types of CKD–MBD. These are bone turnover, bone mineralization and fibrosis quantification assessment. The practiced metric for ABD includes (Fig. 2).21,30,31
- 1.
Bone formation rate less than 97 to 108μm2/mm2/day.
- 2.
Osteoid volume less than 12–15%.
- 3.
No (minimal) fibrosis.
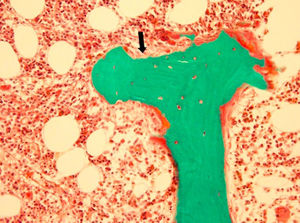
Adynamic bone disease. Depicts a classic bone biopsy featuring minimal osteoid (black arrow) and nearly no activity of osteoblasts or osteoclasts. Image courtesy of Avudai Maran, PhD and Bart Clarke, MD – Biomaterials and Histomorphometry Core Lab, Mayo Clinic, Rochester, MN.
Osteoblastic proteins stimulate mineralization. Fibroblast growth factor-23 FGF-23 is formed by osteoblasts and osteocytes in bone and keep a physiological check on mineral levels by its action on parathyroid, kidney and bone and therefore affecting calcium, phosphorus, calcitriol and parathormone synthesis. It gets activated after its binding to a transmembrane protein, klotho and combination then result in suppression of both PTH and 1,25-dihydroxyvitamin D through downregulating action on NaPi-2a (SLC34A1) and NaPi-2c (SLC34A3) channels in the proximal tubules and distal tubule and suppression of 1α-hydroxylase (thus lower down phosphorous level and calcitriol).32,33
Bone turnover is also affected by many local factors as growth factors and cytokines and interleukins by their action on PTH. Cytokines regulate osteoclastogenesis and osteoprotegerin (OPG), which is a complex system to regulate bone resorption.34 It has a negative association with PTH in ABD. Osteoprotegerin is also a soluble member of the tumor necrosis factor and associated with atherogenesis and endothelial dysfunction.
Clinical featuresThere are no pathognomonic clinical signs of ABD. The two most common symptoms are skeletal pain and fractures.35,36 Although, biopsy proven adynamic bone as a risk factor for fracture has not been demonstrated in the studies but recent guidelines do not recommend routine bone biopsy for making diagnosis.
DiagnosisNo biochemical markers can accurately define ABD. Bone alkaline phosphatase (BAP) is the most useful parameter for bone formation as elevated levels, exclude ABD while low PTH levels distinguishes it from high turnover CKD-MBD.37 Relatively low/normal PTH served as a surrogate marker of low bone turnover and identifies fracture risk in ABD.
The histomorphometric examination of an under calcified bone sample is the gold standard for the diagnosis of ABD.38 The requisites include pre-biopsy in-vivo tetracycline double labelling, amyloid and aluminium staining. Both cortical and trabecular bone should be assessed for static and dynamic parameters to interpret bone metabolism in totality. Favoured site of biopsy is 2cm posterior and 2cm inferior to the anterior iliac crest. The instrument is specifically planned to obtain a core of bone of at least 4–5mm diameter.39
Ectopic calcificationA notable feature of ABD is diminished assimilation of serum calcium into the bone. In a study of 101 hemodialysis patients, 52% had moderate or severe coronary artery calcification (CAC).40 This association was pronounced with older age, higher BMI, inflammation, reduced trabecular volume and with higher osteoprotegerin levels. Asci et al. noted that when only HD patients with CAC were included for analysis, there was a U-shaped relationship between CAC and bone turnover.41 London et al concluded that a high arterial calcification score is defined by bone histo-morphometry suggestive of low bone activity and ABD.42 Studies have shown that anabolic bone stimulating agents such as bone morphogenic protein 7 (BMP-7) or synthetic PTH (1–34) improved bone turnover and skeletal mineralization and decreased calcium deposition in the aorta.43 The mechanism of action of PTH (1-34) seems to be mediated through a combination of both direct and osteopontin effects.
ABD and mortalityMultiple studies have documented relation between low and very high PTH (U-shaped pattern) and risk of sudden death.44–46 In a multi-centric French ARNOS cohort of 1,348 prevalent HD patients, very low PTH levels (<50pg/ml) was associated with poor survival rates (HR: 1.4 (1.07–1.8), p=0.006).47 Also Korean multicenter prospective cohort study of 1,771 incident dialysis patients, revealed association of low serum PTH level (<150pg/ml) and infection-related mortality (hazard ratio [HR], 2.52; 95% CI, 1.06–5.99; p=0.04).48 With the understanding of rise of PTH level with CKD progression, KDIGO suggest maintaining intact PTH levels in the range of approximately two to nine times the upper normal limit for the assay.21 Guidelines gave emphasis for keeping higher targets for PTH as per the stages of CKD and minimise over usage of calcium-based binders and Vitamin D analogues.
Key measures in the management of ABDThe chief management strategy aims to increase PTH synthesis. The following measures may be useful:
- 1.
Switch from calcium-containing phosphate binders to non-calcium, non-aluminium-containing binders:
Sevelamer therapy increases osteoblast surfaces of long bones and bone formation rates and shown to reverse CKD related osteopenia.49 A randomized, prospective, open label study, evaluated 119 HD patients with bone biopsies (59% having ABD at baseline) at the beginning and after 1-year treatment period with sevelamer or calcium compound.50 Sevelamer group resulted in no statistically significant changes in bone turnover or mineralization compared with calcium, but bone formation rate increased and trabecular architecture improved only with sevelamer (p=0.019). In another Japanese study of 40 HD patients who were switched from calcium to sevelamer, showed improvement in bone turnover markers over a period of one year.51
The treatment with lanthanum has also shown beneficial effects in bone histology in various studies. There has been a normalization of the bone histo-morphometric parameters and almost no evolution toward low bone turnover after one year of treatment with lanthanum (compared to calcium carbonate treated patients) in a multi-centric randomized study.52 An additional follow up in a subset of patients (n=20) showed that bone deposition of lanthanum is low after one year.53 Furthermore, there is a slow release of lanthanum from its bone deposits two years after stopping the treatment without any features of aluminium-like bone toxicity. The results of lanthanum treatment were also encouraging in a Japanese prospective dialysis study where two patients with ABD improved after one year of lanthanum treatment.54 The beneficial effects of lanthanum on bone histology persisted for three years.
- 2.
Oral daily dietary calcium intake should be evaluated and reduced to 1200mg. As per current guidelines, daily calcium intake of men aged 19–70 and women aged 19–50 should be up to 1000 and beyond that up to 1200mg.55
- 3.
Stop/reduce active vitamin D medications.
- 4.
Lower dialysate calcium to 1.25mmol/L or below: In a study, fifty-one biopsy-proven ABD patients treated with PD were randomized to treatment with control calcium (1.62mM), or low calcium (1.0mM) dialysate over a 16-month period.56 Bone formation rates increased from18.1±5.6μm2/mm2/day to 159±59.4μm2/mm2/day (p<0.05) in the low calcium group. There was also a reduction in hypercalcemic episodes, which resulted in increased PTH levels and normalization of bone turnover in patients with ABD. In another, randomized, controlled trial of 52 hemodialysis patients over a period of 6 months, all bone parameters in the low calcium dialysate (1.25mmol/L) group were significantly higher than in the group with dialysate calcium of 1.75mmol/L.57 Preventing an overall positive calcium balance and resultant PTH stimulation is useful in these patients. Thus, a low calcium dialysate might be regarded a valuable therapeutic option for ABD patients.
- 5.
A bone biopsy to confirm diagnosis and to assess bone aluminium content and distribution should be done in only indicated patients.58,59
- 6.
Bisphosphonates administration with monitoring for biomarkers.60
- 7.
Calcilytics are currently of undetermined value.61 Ronacaleret, a calcilytic which allows bone formation by serving as a CASR antagonist and increasing endogenous production of PTH showed initial encouraging results in post-menopausal women.62 This agent is yet to be tested in ABD.
- 8.
Teriparatide (PTH (1–34)) as a bone stimulating agent: Teriparatide (recombinant human parathyroid hormone) is an anabolic agent approved for the treatment of patients at high risk for fracture.63 It has been hypothesized that teriparatide would be effective for ABD based on its ability to promote both osteoblast and osteoclast activity. Because teriparatide promotes bone formation, it should also allow the skeleton to recover its function as a reservoir for excess calcium and phosphorus. However, it is not recommended in children with open epiphysis and in those with risk for osteosarcoma. Dose and duration dependent risk of malignant bone tumor was found in rat studies, and therefore, warning for osteosarcoma and hypercalcemia have been added for its usage beyond 2 years. In an open-label, prospective, 6-month observational pilot-study of seven HD patients with ABD and a median iPTH level of 22pg/ml, all patients received 20μg teriparatide/day subcutaneously.64 At 6 months, compared to pre-treatment values, calculated monthly changes in BMD improved significantly in both the lumbar spine and femoral neck (p<0.02). Sumida et al administered teriparatide 56.5μg subcutaneous once weekly to 22 HD patients with serum PTH<60pg/mL.65 BMD at lumbar spine increased by 3.3±1.9% (p<0.05) and 3.0±1.8% at 24 and 48 weeks respectively, without significant changes in femoral neck and distal radius BMD. Further clinical studies are needed to establish teriparatide as a therapeutic option for dialysis patients with ABD.
- 9.
Abaloparatide: a PTH-related peptide analogue that specifically activates PTH receptor type I pathway was developed as another anabolic drug.66 In a phase 3 Abaloparatide Comparator Trial In Vertebral Endpoints (ACTIVE) trial, treatment with abaloparatide for 18 months significantly increased BMD and decreased the risk of vertebral, nonvertebral, clinical, and major osteoporotic fractures compared with a placebo.67 Abaloparatide is approved in the US for the treatment of postmenopausal women with osteoporosis at high risk for fracture. However, potential benefits of this drug in ABD needs to be explored.
- 10.
Romosozumab: Romosozumab is a monoclonal antibody that binds and inhibits sclerostin. (The wingless in Drosophila and integrated in vertebrate (Wnt) signaling pathway is a crucial regulator of osteoblast recruitment.68 Sclerostin is an endogenous inhibitor of this pathway, thereby inhibiting osteoblast recruitment and decreasing bone formation.69 The resultant effects include increase in bone formation and decrease in bone resorption. The beneficial effects of this drug compared to placebo in increasing BMD and reducing vertebral fractures in postmenopausal osteoporotic women have been seen in clinical studies.70 Identical triumph in using romosozumab to improve bone density in patients with ABD, although might be promising in coming years, but it's important not to overlook the potential risk of myocardial infarction, stroke, and cardiovascular death and should not be initiated in patient with such risk factors.
ABD is a complex disease process which is associated with increased morbidity and mortality. Prevention through the judicious use of calcium containing phosphate binders and antiparathyroid agents is likely to be more effective than the treatment of established disease. However, there are promising therapeutic interventions whose role needs to be established in appropriate trials.
Compliance with ethical standardsThis article does not contain any studies with human participants or animals performed by any of the authors.
Informed consentNot applicable for this review article.
Financial disclosureNone.
Conflicts of interestNone.