Adenovirus infection is a self-limiting disease in healthy adults, however in immunodeficient hosts such as transplant recipients they may cause severe or fatal illness. Kidney involvement in transplant recipients more frequently manifests as hemorrhagic cystitis and tubulo-interstitial nephritis.1,2 The incidence of asymptomatic viremia has been observed in up to 11% within the first year following solid organ transplantation.3
We present a case of a 40-year-old man who underwent kidney transplantation (KT) of low immunological risk from a living unrelated donor. Induction therapy included basiliximab 20mg (D0 and D4 after surgery), methylprednisolone (250mg, 125mg, 62.5mg on D1, D2 and D3, respectively) followed by prednisolone at a daily dose of 20mg, tacrolimus and mycophenolate de mofetil (1000mg every 12h)—both started a week before KT. On D7 he referred dysuria and urinalysis revealed leucocyturia which prompted ceftriaxone start. He was discharged on D8 after KT, with a serum creatinine (SCr) level of 1.6mg/dL and fluctuating tacrolimus through levels between 5.0 and 10.7ng/mL. Maintenance immunosuppression was achieved with tacrolimus (6.5mg/day), prednisolone (15mg/day) and mycophenolate mofetil (1000mg every 12h). Additional chemoprophylaxis was completed with cothrimoxazol 480mg/daily.
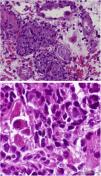
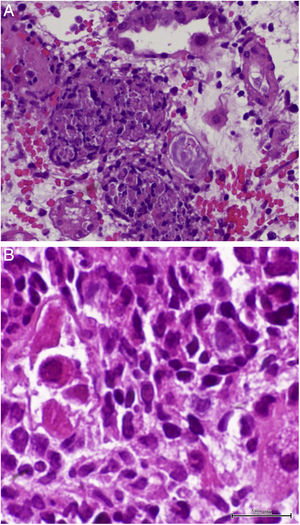
On D17 after KT, the patient presented to the emergency department with fever, diarrhea and renal dysfunction (SCr 2.0mg/dL). Spot urinalysis documented microscopic hematuria and leucocyturia. Graft doppler ultrasonography was normal. He was diagnosed with pyelonephritis and intravenous ceftriaxone was started. His toddler developed superior respiratory infection a couple days before. Despite the start of antibiotic, fever persisted and SCr level increased up to 2.2mg/dL. Initial blood, stool and urine cultures, as well as blood polymerase chain reaction (PCR) for cytomegalovirus (CMV) and polyomavirus, stool PCR for rotavirus, noravirus, astrovirus and clostridium were negative. On D23, the antibiotic was stopped and an allograft biopsy was performed, revealing interstitial nephritis with granulomatous-like appearance and ground glass tubular epithelial cell nuclei suggesting viral inclusions (Fig. 1). Immunoperoxidase stain was negative for polyomavirus and immunofluorescence was negative for C4d. The result of serum, urine and kidney tissue PCR was positive for adenovirus. These findings prompted mycophenolate mofetil withhold plus intravenous ganciclovir (2.5mg/kg for 13 days) and intravenous immunoglobulin (IVIG 2mg/kg for 4 days) start. Fever remitted in four days and allograft function improved (SCr 1.9mg/dL on D34 after surgery). Upgrading dosing of mycophenolate mophetil was slowly introduced two weeks after kidney biopsy and valganciclovir (450mg twice daily) was maintained until serum adenovirus PCR became negative after 54 days of treatment. Allograft function slowly improved and SCr stabilized at 1.7mg/dL six months after transplantation.
Native kidneys’ acute interstitial nephritis (AIN) is more commonly associated with drugs. In contrast, drug-induced AIN is rarely reported in kidney grafts, possibly due to the use of corticosteroids in maintenance immunosuppression strategy. Lymphocytic interstitial infiltrates can be observed in any type of acute renal allograft dysfunction, including calcineurin inhibitors nephrotoxicity, acute tubular necrosis and viral infections, as well as in normally functioning renal allografts.4 Diagnosis can be challenging as AIN and acute cellular rejection share histological features, namely a primarily composed lymphocyte interstitial infiltrate, with prominent number of eosinophils.5 In this case report, patient's therapy included many potential AIN inducers, such as antibiotics, and tacrolimus fluctuating through levels could portend an acute rejection.
Granulomatous AIN can be found with mycobacterial, fungal, and viral infections, such as adenovirus, human immunodeficiency virus, CMV and polyomavirus, which were all negative in our patient, except for adenovirus. Kidney biopsy in adenovirus infection often shows interstitial nephritis with viral inclusions in tubular epithelial cells. Granulomatous interstitial nephritis with neutrophilic inflammation, red blood cells in tubules, and parenchymal necrosis is also more frequent in adenovirus than polyomavirus or CMV infection.6 Another supporting feature of adenovirus-induced AIN is the presence of granulomas surrounding tubules.7 In this case, the presence of enlarged tubular epithelial cells with groundglass homogenous intranuclear viral inclusions, suggested a viral etiology and the distinctive pattern of granulomatous infiltrate helped discriminate acute rejection from adenovirus infection.
Adenovirus infection in KT recipients should be considered whenever a KT recipient presents with unexplained voiding dysfunction, hematuria, and sterile pyuria. There is uncertainty regarding monitoring of this virus, but early diagnosis with reduction of immunosuppression is essential to virus clearance and allograft function.8
Unlike CMV infection, there is no consensual therapeutic approach for AIN associated with adenovirus infection. Treatment strategies are based on case reports and series of cases, which include immunosuppression tapering and specific antiviral therapy, such as IVIG, pulse–dose steroids, cidofovir, ribavirin or valganciclovir, in varying combinations, with or without reduction in immunosuppression.9,10 Given the inherent toxicity associated to most of these drugs, we report the successful treatment of adenovirus-induced AIN with ganciclovir, IVIG, imunossupression reduction and valganciclovir extended treatment.