The epidemiology of diabetes mellitus (DM) has changed during recent years. DM has been a growing epidemia in recent decades, confirming the predictions of World Health Report in 1997 that estimated progressive growth of the disease during the following 20 years.1 In year 2012, the prevalence of diabetes in USA was 14% (9% of cases with known diagnosis), but it was especially remarkable and alarming that there was a 38% population in situation of prediabetes.2 If such a trend continues by 2050, one out of every 3 adults in the USA will be diabetic.3 The increase in the prevalence of DM has occurred especially at the expense of DM type 2 (DM-2), due to changes in lifestyle and increased obesity.4 In USA, the health cost of DM in 2012 amounted to 245,000 million dollars, including the repercussion derived from the lack of productivity of patient with complications. Fortunately, although between 1990 and 2010 the population of diabetics in USA grew by 27%, the percentage of complications linked to DM decreased: amputations, from 22.6% to 18.8%; terminal renal failure, from 13.7% to 6.1%; myocardial infarction, from 3.8% to 1.8%; and stroke, from 3.1% to 1.5%,5 this is probably due to an improved diagnosis and care of both DM as of its complications.
The globalization of DM is a world health problem, with a increase in the Incidence and prevalence that include variants such as gestational diabetes and DM type MODY (Maturity Onset Diabetes of the Young), that is, diabetes of mature age that occurs in the young.6,7 In Spain, the Di@bet.es study, carried out in 100 centers with wide geographical distribution, found some disorder of the hydrocarbon metabolism in about 30% of the population studied.8 The prevalence of DM, adjusted for age and gender, was 13.8% (95% CI: 12.8–14.7%), and a 6% (95% CI: 5.4–6.7%) of the population was not aware of being diabetic. The socioeconomic impact of DM and its complications in our country is important, with an estimated global cost of € 2132/patient/year if micro- and macrovascular complications are present.9 A reduction in Chronic kidney disease stage 5 (CKD-5) may save between 15 and 25 million euros in 3 years in the Canary Islands.10
The presence of albuminuria above the values considered as normal and the progression toward proteinuria have been the most common forms of clinical expression of diabetic nephropathy However, in recent years there has been a growing description of the progression toward renal failure without developing proteinuria11 which has led to postulate the existence of a “non-proteinuric phenotype”12 Tervaert et al.13 proposed in 2010 a new histopathological classification of renal lesions in DM, insisting on the finding of tubulo-interstitial and/or vascular lesions in absence of glomerular lesions as an initial form of renal involvement. All this leads to a change from the classic concept of diabetic nephropathy to a more generic concept of “diabetic kidney disease”.4
Prevention of diabetic kidney disease and cardiovascular disease in DMThe current Clinical Practice Guidelines and the Documents for the prevention and management of Diabetes Mellitus (DM) and Diabetic Kidney Disease (DKD) include several elements: adequate control of glycemia and blood pressure, intervention to reduce renal and CV risk factors, healthy life style with exercise appropriate to the situation of each patient, adequate diet in relationship to sodium and protein intake, adequate intake of carbohydrate, abandonment of smoking, lipid monitoring, treatment with Inhibitors of Renin Angiotensin-Aldosterone System (RAAS-I), optimization of o hypoglycaemic drugs to the renal function of each patient throughout the evolution of CKD and coordinated multifactorial and multidisciplinary control to prevent the progression of micro and macrovascular damage.14–21
Despite all these recommendations and strategies, DKD remains the first cause of advanced CKD that will require Renal Replacement Treatment (RRT).
The need to reduce the progression of microvascular and macrovascular damage in DM has led in recent years to multicenter studies including vascular and renal targets and the search for new agents that have been evaluated in numerous clinical trials, as well as the use of specific therapies trying to stop the progression of vascular and renal damage.
There are numerous experimental studies on new drugs that have been considered candidates to be subsequently translated to the clinical scenario but few have demonstrated a positive impact on the management of DM and its complications.
New hypoglycemic agents: pleiotropic effects beyond glycemic controlDespite the plethora of studies on new drugs and the effectiveness demonstrated by many of them, we are aware that the control of blood glucose, BP and proteinuria are not sufficient, in many of our patients with DM, to prevent the occurrence of DKD and the progression of CKD.
For this reason, the pharmaceutical industry and researchers are vigorously trying to find new alternatives, in such a way that the investigation of new molecules is one of the most intense development fields in recent years, as shown by the growing number of clinical trials and other studies. Currently there are about 40 Studies with new molecules (www.clinicaltrials.gov). The analysis of all these new molecules would far exceed the extension allowed for this manuscript and the limits accepted by the journal. Thus we will focus on most recent drugs for the management of hyperglycemia and its effects related and nonrelated to its hypoglycemic effect.
- 1.
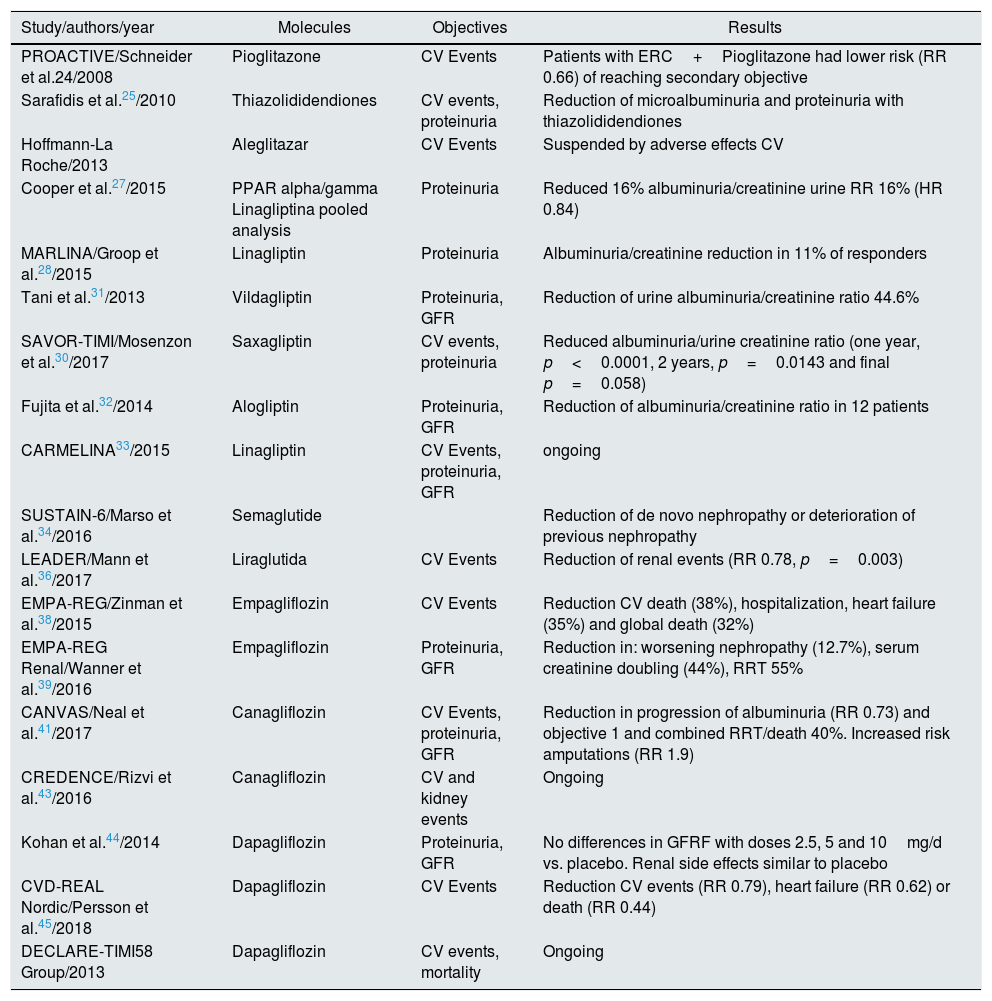
Some hypoglycemic agents have shown to have renoprotective effects, independently of its effect on glucose concentration (see Tables 1 and 2). Numerous studies with agonists of receptor activated by proliferators of the Gamma perixosomes, (PPAR-δ) or thiazolidinediones (TZD) have shown to be able to reduce albuminuria.22,23 The post hoc analysis of the study PROACTIVE (Prospective Pioglitazone Clinical Trail in Macro-Vascular Events), which included 5238 patients with DM2 and macrovascular disease, showed a greater GFR reduction in the group treated with pioglitazone than with placebo (difference between groups, 0.8mL/min/1.73m2).24 A meta-analysis that included 15 studies with thiazolidinediones, (10 with pioglitazone and 5 with rosiglitazone), including 2860 patients, found significant reductions in albuminuria. However, the harmful cardiovascular effects such as increased hydrosaline retention, have limited the use of these agents.25
Table 1.Some pilot studies and recent multicenter studies being analyzed.
Study/authors/year Molecules Objectives Results PROACTIVE/Schneider et al.24/2008 Pioglitazone CV Events Patients with ERC+Pioglitazone had lower risk (RR 0.66) of reaching secondary objective Sarafidis et al.25/2010 Thiazolididendiones CV events, proteinuria Reduction of microalbuminuria and proteinuria with thiazolididendiones Hoffmann-La Roche/2013 Aleglitazar CV Events Suspended by adverse effects CV Cooper et al.27/2015 PPAR alpha/gamma Linagliptina pooled analysis Proteinuria Reduced 16% albuminuria/creatinine urine RR 16% (HR 0.84) MARLINA/Groop et al.28/2015 Linagliptin Proteinuria Albuminuria/creatinine reduction in 11% of responders Tani et al.31/2013 Vildagliptin Proteinuria, GFR Reduction of urine albuminuria/creatinine ratio 44.6% SAVOR-TIMI/Mosenzon et al.30/2017 Saxagliptin CV events, proteinuria Reduced albuminuria/urine creatinine ratio (one year, p<0.0001, 2 years, p=0.0143 and final p=0.058) Fujita et al.32/2014 Alogliptin Proteinuria, GFR Reduction of albuminuria/creatinine ratio in 12 patients CARMELINA33/2015 Linagliptin CV Events, proteinuria, GFR ongoing SUSTAIN-6/Marso et al.34/2016 Semaglutide Reduction of de novo nephropathy or deterioration of previous nephropathy LEADER/Mann et al.36/2017 Liraglutida CV Events Reduction of renal events (RR 0.78, p=0.003) EMPA-REG/Zinman et al.38/2015 Empagliflozin CV Events Reduction CV death (38%), hospitalization, heart failure (35%) and global death (32%) EMPA-REG Renal/Wanner et al.39/2016 Empagliflozin Proteinuria, GFR Reduction in: worsening nephropathy (12.7%), serum creatinine doubling (44%), RRT 55% CANVAS/Neal et al.41/2017 Canagliflozin CV Events, proteinuria, GFR Reduction in progression of albuminuria (RR 0.73) and objective 1 and combined RRT/death 40%. Increased risk amputations (RR 1.9) CREDENCE/Rizvi et al.43/2016 Canagliflozin CV and kidney events Ongoing Kohan et al.44/2014 Dapagliflozin Proteinuria, GFR No differences in GFRF with doses 2.5, 5 and 10mg/d vs. placebo. Renal side effects similar to placebo CVD-REAL Nordic/Persson et al.45/2018 Dapagliflozin CV Events Reduction CV events (RR 0.79), heart failure (RR 0.62) or death (RR 0.44) DECLARE-TIMI58 Group/2013 Dapagliflozin CV events, mortality Ongoing CV: cardiovascular; CKD: chronic kidney disease; GFR: glomerular filtration rate; HR: hazard ratio; PPAR: receptors activated by perixosome proliferators; RR: relative risk; RRT: renal replacement therapy.
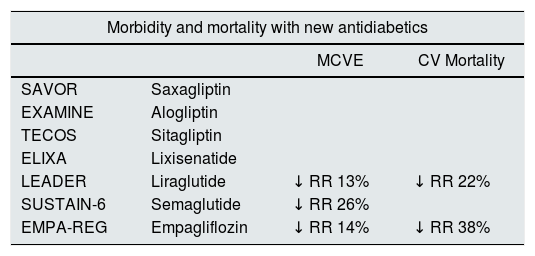
Table 2.Cardiovascular morbidity and mortality and renal events with some new hypoglycaemic agents.
Morbidity and mortality with new antidiabetics MCVE CV Mortality SAVOR Saxagliptin EXAMINE Alogliptin TECOS Sitagliptin ELIXA Lixisenatide LEADER Liraglutide ↓ RR 13% ↓ RR 22% SUSTAIN-6 Semaglutide ↓ RR 26% EMPA-REG Empagliflozin ↓ RR 14% ↓ RR 38% Kidney events with new antidiabetics Kidney events with new antidiabetics MAB progression Duplication of Serum creatinine Initiation of RRT LEADER ↓ RR 22% SUSTAIN-6a ↓ RR 14% EMPA-REG ↓ RR 14% ↓ RR 14% ↓ RR 14% CV: cardiovascular; MAB: microalbuminuria; MCVE: major cardiovascular episode; RR: relative risk; RRT: renal replacement therapy.
Aleglitazar is an agent with double effect PPAR α and δ, that should have anti-inflammatory effects, improvement of both lipid profile and blood glucose level. The clinical trial that was intended shows these effects, as well as a potential reduction of proteinuria, but was suspended prematurely due also to adverse CV effects, similar to those observed in the previous studies with TZD.26
- 2.
The introduction of Dipeptidyl-Peptidase 4 (iDPP4) inhibitors has been a revolution in the management of hyperglycemia in DM, due to the “Incretin” effect of these drugs. DPP-4 is the enzyme that regulates the degradation of Glucagon-like-peptide 1 (GLP-1), an incretin released in the intestine in response to food intake to stimulate insulin and suppress glucagon production. Numerous studies have tried to show the beneficial vascular and renal effects of these hypoglycemic agents,(vildagliptin, sitagliptin, saxagliptin, alogliptin, linagliptin and teneligliptin). It is not the objective of this review to analyze all the very variable results and we will only refer to some of them.
A combined analysis of four phase III studies that included 217 patients with DM2 and diabetic nephropathy, previously treated with RAAS blockers, showed beneficial effects of linagliptin, (the only iDPP-4 together with teneligliptin that does not require dose adjustment in renal failure), in reducing proteinuria by 32%, regardless of the effect on HbA1c.27
Subsequently, the MARLINA study (Microalbuminuria and Renal Efficacy with Linagliptin)28 tested the efficacy of single dose of 5mg/day of linagliptin in patients with DM2 and nephropathy. Linagliptin was only able to slow down the albuminuria in the group of patients called “responders”, (11.1% of the Patients), but not in the overall of patients treated with the drug.29 The SAVOR-TIMI study, which included 16,493 patients with DM2, showed reduction of albuminuria in only 11.8% of patients who received saxagliptin and had available the albumin/creatinine ratio in urine.30 The study by Tani et al.31 with vildagliptin showed a reduction of albuminuria in patients with DM2, but only included 47 patients. The study by Fujita et al., with a cross-over design of sitagliptin/alogliptin, showed a reduction of albuminuria in the group treated with alogliptin, but only included 12 patients.
There are several studies in progress on the CV and renal effects of the iDPP-4 drugs. The potential benefits of linagliptin on vascular and renal protection in DM2 patients with nephropathy and high CV risk are being evaluated in the CARMELINE study.33 The clinical trial has completed the follow-up and the results are expected after October 2018.
- 3.
The introduction of other drugs with incretin effect, the agonists of GLP-1 receptor, (exenatide, liraglutide, lixisenatide, albiglutide, semaglutide, dulaglitide and others), has also represented a significant change in the possibilities of hyperglycemic control. There numerous studies underway to evaluate the possible vascular and renal benefits of these drugs.
The SUSTAIN 6 study (Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes) has included 3297 patients with DM2, randomized to receive standard treatment or semaglutide 0.5mg or 1mg/week subcutaneously, for 104 weeks. Out of the 1649 treated with semaglutide, 108 (6.6%) achieved the primary composite goal (CV death: nonfatal myocardial infarction – non fatal stroke), vs. 146 of the 1649 included in the standard treatment group (8.9%), RR 0.74, p<0.001. The authors concluded that semaglutide significantly decreased CV risk in patients with DM2 with high CV risk. The study also showed that the percentage of worsening of the preexisting nephropathy was less in the group of patients treated with semaglutide.34,35
The results of the LEADER study (Liraglutide and Renal Outcomes in type 2 DM) have just been published. In this study, 9340 patients were randomized, received liraglutide in dayly subcutaneous dose vs. placebo, added to conventional treatment, and patients were followed for 3.84 years. The number of events of the combined renal target was lower in the group treated with liraglutide than in the placebo group (268 out of 4668 patients vs. 337 out of 4672; RR 0.78, p=0.003). Persistent proteinuria was detected in fewer patients receiving liraglutide than placebo(161 vs. 215 patients, RR, 0.74, p=0.004). The results show that in DM-2 patients the addition of liraglutide to conventional treatment reduces the percentage with progression of kidney damage.36
More recently it has been published the results of the EXSCEL study (Effects of Once-Weekly Exenatide on cardiovascular Outcomes in type 2 diabetes). In such study, 14,752 patients with DM-2 were randomized to receive prolonged release exenatide, 2mg weekly subcutaneous injection, vs placebo, added to conventional treatment. The mean follow-up was 3.2 years. A 11.4% of patients (n=839) on Exenatide achieved a primary goal composed of death of cardiovascular cause, non-fatal myocardial infarction or non-fatal cerebrovascular accident vs. a 12.2% (905 patients) in the placebo group (RR 0.91, p<0.001 for non-inferiority).37
The Na-Glucose Co-transporter Inhibitors Type 2 (iSGLT2) represent one of the most promising therapeutic novelties in the management of DM.
The various SLGT receptors are widely distributed but with variations between the various organs. The SGLT2 are mainly located in the kidney. In the kidney, the glucose filtered at the glomerular level is totally reabsorbed by the tubules through SGLT2. The ISGLT2 act on the proximal renal tubules preventing the tubular reabsorption of glucose and favoring its elimination in urine.
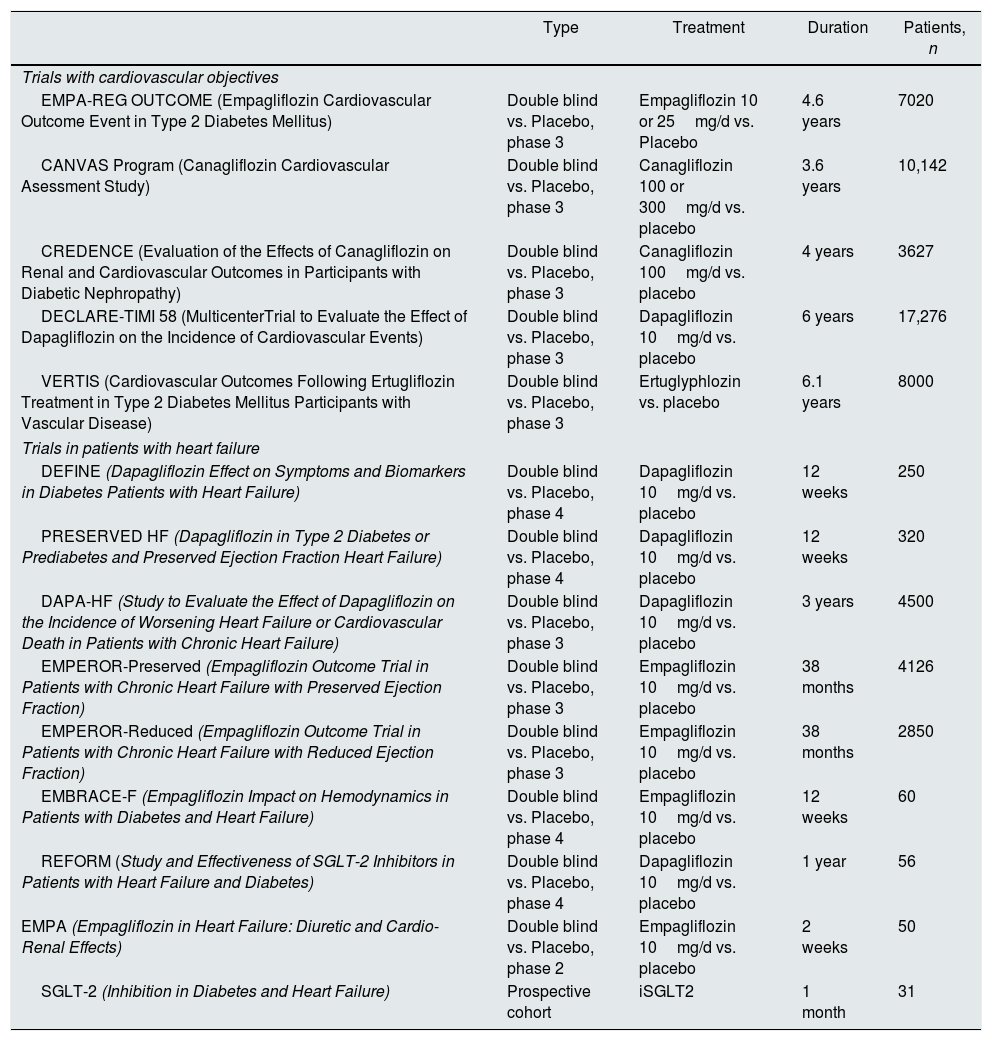
At the present time we have the possibility of using canagliflozin, dapagliflozin and empagliflozin, and other iSGLT2 are under development (see Table 3).
Clinical trials and studies with sodium-glucose cotransporter type 2 inhibitors in execution.
| Type | Treatment | Duration | Patients, n | |
|---|---|---|---|---|
| Trials with cardiovascular objectives | ||||
| EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event in Type 2 Diabetes Mellitus) | Double blind vs. Placebo, phase 3 | Empagliflozin 10 or 25mg/d vs. Placebo | 4.6 years | 7020 |
| CANVAS Program (Canagliflozin Cardiovascular Asessment Study) | Double blind vs. Placebo, phase 3 | Canagliflozin 100 or 300mg/d vs. placebo | 3.6 years | 10,142 |
| CREDENCE (Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants with Diabetic Nephropathy) | Double blind vs. Placebo, phase 3 | Canagliflozin 100mg/d vs. placebo | 4 years | 3627 |
| DECLARE-TIMI 58 (MulticenterTrial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events) | Double blind vs. Placebo, phase 3 | Dapagliflozin 10mg/d vs. placebo | 6 years | 17,276 |
| VERTIS (Cardiovascular Outcomes Following Ertugliflozin Treatment in Type 2 Diabetes Mellitus Participants with Vascular Disease) | Double blind vs. Placebo, phase 3 | Ertuglyphlozin vs. placebo | 6.1 years | 8000 |
| Trials in patients with heart failure | ||||
| DEFINE (Dapagliflozin Effect on Symptoms and Biomarkers in Diabetes Patients with Heart Failure) | Double blind vs. Placebo, phase 4 | Dapagliflozin 10mg/d vs. placebo | 12 weeks | 250 |
| PRESERVED HF (Dapagliflozin in Type 2 Diabetes or Prediabetes and Preserved Ejection Fraction Heart Failure) | Double blind vs. Placebo, phase 4 | Dapagliflozin 10mg/d vs. placebo | 12 weeks | 320 |
| DAPA-HF (Study to Evaluate the Effect of Dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients with Chronic Heart Failure) | Double blind vs. Placebo, phase 3 | Dapagliflozin 10mg/d vs. placebo | 3 years | 4500 |
| EMPEROR-Preserved (Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction) | Double blind vs. Placebo, phase 3 | Empagliflozin 10mg/d vs. placebo | 38 months | 4126 |
| EMPEROR-Reduced (Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Reduced Ejection Fraction) | Double blind vs. Placebo, phase 3 | Empagliflozin 10mg/d vs. placebo | 38 months | 2850 |
| EMBRACE-F (Empagliflozin Impact on Hemodynamics in Patients with Diabetes and Heart Failure) | Double blind vs. Placebo, phase 4 | Empagliflozin 10mg/d vs. placebo | 12 weeks | 60 |
| REFORM (Study and Effectiveness of SGLT-2 Inhibitors in Patients with Heart Failure and Diabetes) | Double blind vs. Placebo, phase 4 | Dapagliflozin 10mg/d vs. placebo | 1 year | 56 |
| EMPA (Empagliflozin in Heart Failure: Diuretic and Cardio-Renal Effects) | Double blind vs. Placebo, phase 2 | Empagliflozin 10mg/d vs. placebo | 2 weeks | 50 |
| SGLT-2 (Inhibition in Diabetes and Heart Failure) | Prospective cohort | iSGLT2 | 1 month | 31 |
iSGLT2: inhibitors of the sodium-glucose cotransporter type 2. Modified from Lytvyn et al.47
The EMPA-REG study37 included 7028 patients with DM2, of whom 2345 received empagliflozin 10mg/day; 2348 received empagliflozin 25mg/day and 2333 were the placebo group. The study variable was a combined of death from CV cause, non-fatal myocardial infarction and stroke not mortal. The secondary variable was a composite of the main variable plus hospitalization.
The study showed a significant decrease in episodes in all patients on empagliflozin (490 from 4687 patients, 10.5%) versus placebo (282 in 2333, 12.1%), RR 0.86, p<0.001 for non-inferiority and p=0.04 for superiority. Analysis of the two groups of 10 and 25mg separately, showed no significant differences, with RR of 0.85 for the group treated with 10mg/day and 0.86 for the group on 25mg/day.
The secondary objective was achieved in 599 of 4687 patients (12.8%) in the empagliflozin group and in 333 of 2333 patients (14.3%) in the placebo group, RR 0.62, p<0.001. After publication of the study EMPAREG, the results of the EMPA-REG Renal Study (95) were analyzed. These were focused on the renal effects of treatment with empagliflozin at doses of 10 or 25mg/day in the 7028 patients with DM2 and various degrees of kidney disease. The final analysis have shown that empagliflozin, both at doses of 10 and 25mg/day, was associated with a significant reduction of renal targets: progression from micro to macroalbuminuria (proteinuria), reduction of the risk of a composite goal of serum creatinine duplication, initiation of renal replacement therapy, death of patient. The results of the study suggest beneficial CV effects beyond the action on glycemic control. Results of new additional studies were expected in March 2018 to corroborate if this is a “class effect” and occurs with other inhibitors of SLGT2, or it is specific of empagliflozin.
The results EMPA-REG study referring to empagliflozin's effects in patients with established CV disease and chronic kidney disease (GFR <60mL/min/1.73m2) have been reported more recently. These results have shown a decrease in the relative risk of mortality (RR 0.71) of 29% for all categories of GFR and albuminuria, including patients with GFR <60mL/min/1.73m2.38–40
Very recently we have known the results of the CANVAS Program with Canagliflozin. The CANVAS program integrates data from two clinical trials including 10,142 patients with DM2 and high CV risk. The participants in each study were randomized to receive treatment with canagliflozin 100 or 300mg/day orally, or placebo. The mean follow-up was 188.2 weeks. The main objective was a c composite of CV death, non-fatal myocardial infarction, or non-fatal stroke. The mean age was 63.3 years and the mean duration of DM was 13.5 years. A 65.6% of patients had a previous history of CV disease. The main objective was reached in 26.9 vs. 31.5 of participants per 1000 patients/year; RR, 0.86; p<0.001 for non-inferiority, p=0.02 for superiority. The renal effects did not show statistical significance, but it showed a possible benefit of canagliflozin on the progression of albuminuria (RR, 0.73) and the 40% reduction in a composite goal of GFR, need for RRT, death of renal cause (RR, 0.60, 95%, CI: 0.47–0.77).
Unfortunately, the results also showed an increase in the risk of distal amputations (6.3 vs. 3.4 participants per 1000 patients/year; RR 1.97; 95% CI, 1.41–2.75). However, information explaining the cause of such a finding is lacking. Although in the multivariate analysis the risk factors for amputations were: antecedents of previous amputations (HR: 20.9 [14.2–30.8]), peripheral vascular disease (HR: 3.1 [2.2–4.5]), male sex (HR: 2.4 [1.6–3.5]), autonomic neuropathy (HR: 2.1 [1.4– 2.6]), HbA1c>8% (HR: 1.9 [1.4– 2.6]), treatment with canagliflozin (HR: 1.8 [1.3–2.5]), and presence of cardiovascular disease (HR: 1.5 [1.0–2.3]).
The authors have concluded that canagliflozin decreases the risk of CV episodes, although with an increased risk of distal amputations.39 The publication of renal effects and long-term CVa42,43 of the CREDENCE study is pending. CREDENCE is Randomized, Double-blind, Placebo-controlled, Parallel-group, Two-arm, Multicenter Study to Assess the Efficacy of Canagliflozin on End Stage Kidney Disease and Vascular Death in Subjects with Type 2 Diabetes Mellitus and Nephropathy. (98). This is the first clinical trial with iSGLT2 that includes a primary renal endpoint (initiation of renal replacement therapy, doubling of serum creatinine and renal or cardiovascular death). Includes patients with albuminuria >300mg and eGFR between 30 and 90mL/min/1.73m2.
The renal and CV effects of dapagliflozin are also being studied in numerous trials and studies. Kohan et al. have published a meta-analysis of 12 studies including 4545 patients with DM2 with durations greater than 24 weeks. He has reported transient decrease in the GFR, and no change after 108 weeks of follow-up and with few adverse effects.44
Also recently we have known the results of the Study CVD-REAL World Nordic. This is a global analysis performed by the national registries of Denmark, Norway and Sweden, dividing patients into two groups: those treated de novo with either dapagliflozin (n=10,227) or with iDPP-4 (n=30,681), the average age was 62 years and 23% had history of CV disease. As compared to iDPP-4, Dapagliflozin was associated with lower risk of major CV episodes (RR 0.79), heart failure (RR 0.62) or mortality from any cause (RR 0.44). Specify kidney targets are not specified in this analysis.45
The results of the study DECLARE (DECLARE-TIMI58), which evaluates the incidence of CV events: CV mortality, myocardial infarction or cerebral vascular accident, in patients with DM2 treated with dapagliflozin 10 mg/day have been very recently published. Dapagliflozin did not result in a higher or lower rate of MACE than placebo but did result in a lower rate of cardiovascular death or hospitalizations for heart failure. 46
Table 2 summarizes the morbidity and mortality and renal events with new hypoglycemic drugs. Table 3 summarizes clinical trials and ongoing studies with iSGLT2, modified from Lytvyn et al.47
Taken together the results so far available from studies withGLP1 agonists and iSGLT2 (47), we might assume that both can reduce the incidence of ERD. However, important limitations persist: they must be designed studies with specifically “renal” main objectives. Secondly, the results regarding nephroprotection withGLP1 agonists, although promising, are limited, so it is an open question,that must be answered in future trials and studies. The nephrologists have to adcquire experience in its management and the possible “class effects” of both types of drugs. Likewise, the possible CV benefits should be clarified as well as Renal diseases in the general population with DM and not only high-risk CV groups included in the studies so far carried out. Finally, the high cost barrier of the drugs will have to be balanced with the possible benefits obtained.
ConclusionsDM continues to be the most frequent cause of advanced CKD in our environment, despite the stabilization in its frequency as a cause of stage 5 nephropathy in the last years. We are convinced that the results obtained with the drugs described here and others that will appear gradually, will help us to manage more adequately the patient with DM and to face with greater probabilities of success the future in the prevention and treatment of vascular and renal disease in DM. We believe that these new molecules, handled early and adequately to the renal function, they will contribute to decrease the incidence and prevalence of DRD, together with the application of other well-known classic standards.
Please cite this article as: Martinez-Castelao A, Soler MJ, Navarro-González JF, Górriz JL. ¿Serán las nuevas moléculas efectivas en protección renal y cardiovascular en la diabetes mellitus y la enfermedad renal diabética? Nefrologia. 2019;39:3–10.