Vitamin D deficiency has been linked to many different pathologies, especially with morbimortality in patients with chronic kidney disease. The progressive loss of renal function leads to calcitriol deficiency and homeostatic changes in calcium, phosphorus, FGF-23 and PTH, among others. All these changes can also influence vitamin D receptor (VDR) activation and the development of secondary hyperparathyroidism (SHPT). The biologic actions of both vitamin D and its synthetic analogues are mediated by binding to the same VDR, acting on different genes. There is a narrow relationship between low levels of calcitriol and SHPT. The combined approach of VDR activation and phosphate restriction, among others, plays an important role in the early treatment of the chronic kidney disease-mineral and bone disorder (CKD-MBD). The Spanish Society of Nephrology, in order to reduce the uniform and significant association with CKD-associated mortality, calcidiol and high phosphate levels suggests normalization of phosphate as well as calcidiol levels in both CKD and dialysis patients. Moreover, it considers that, in addition to selective/non selective activation of VDR for the prevention and treatment of SHPT, VDR could be activated in dialysis patients by native vitamin D or even low paricalcitol doses, independently of PTH levels, as some cohort studies and a recent metaanalysis have found an association between treatment with active vitamin D and decreased mortality in patients with CKD. In general it is considered reasonable to use all this information to individualise decision making.
El déficit de vitamina D se asocia a distintas patologías, siendo especialmente significativa con la morbimortalidad en pacientes con enfermedad renal crónica (ERC). La pérdida progresiva de la función renal conduce a una reducción de calcitriol y alteración de la homeostasis de calcio, fósforo, FGF-23 y PTH, entre otros, los cuales influyen a su vez sobre la activación del receptor de vitamina D (RVD) y el desarrollo de hiperparatiroidismo secundario (HPS). El RVD media las acciones biológicas tanto de la vitamina D como de sus análogos sintéticos, actuando sobre distintos genes; existe una estrecha asociación entre niveles bajos de calcitriol y la prevalencia del HPS. Así, la activación de los RVD y la restricción de fósforo, entre otros, desempeñan un papel importante en el tratamiento de la «alteración óseo-mineral asociada a la ERC». La Sociedad Española de Nefrología, dada la uniforme e importante asociación con mortalidad y niveles altos de fósforo, aconseja su normalización, así como la de los niveles de calcidiol. Igualmente considera que, aparte de la utilización de activadores selectivos/no selectivos de RVD para la prevención y tratamiento del HPS, se podría asegurar la activación de los RVD en pacientes en diálisis, con vitamina D nativa o incluso bajas dosis de paricalcitol, independientemente de la PTH, dado que algunos estudios de cohortes y un metaanálisis reciente han observado una asociación entre el tratamiento con vitamina D activa y la disminución de la mortalidad en pacientes con ERC. En general, se considera que es razonable utilizar toda esta información para individualizar la toma de decisiones.
INTRODUCTION
This manuscript is the result of a consensus meeting held by Spanish specialists on the effects of paricalcitol on bone/mineral metabolism. A second document, which is currently being drafted, will focus on its pleiotropic effects.
Vitamin D deficiency has been associated with different diseases such as hypertension, diabetes, cancer, or heart failure in the general population, but its association with morbidity and mortality in patients with chronic kidney disease (CKD) is particularly significant and homogeneous. Understanding of the role played by the vitamin D receptor (VDR) and the effects of its activation has changed dramatically in recent years. Thus, several studies have analyzed the differential effects of different VDR activators (agonists), the new concept of selective VDR activation and the spectrum of vitamin D-mediated effects has been expanded to its pleiotropic extraskeletal effects and its participation in the so-called cardiorenal syndrome.1-4
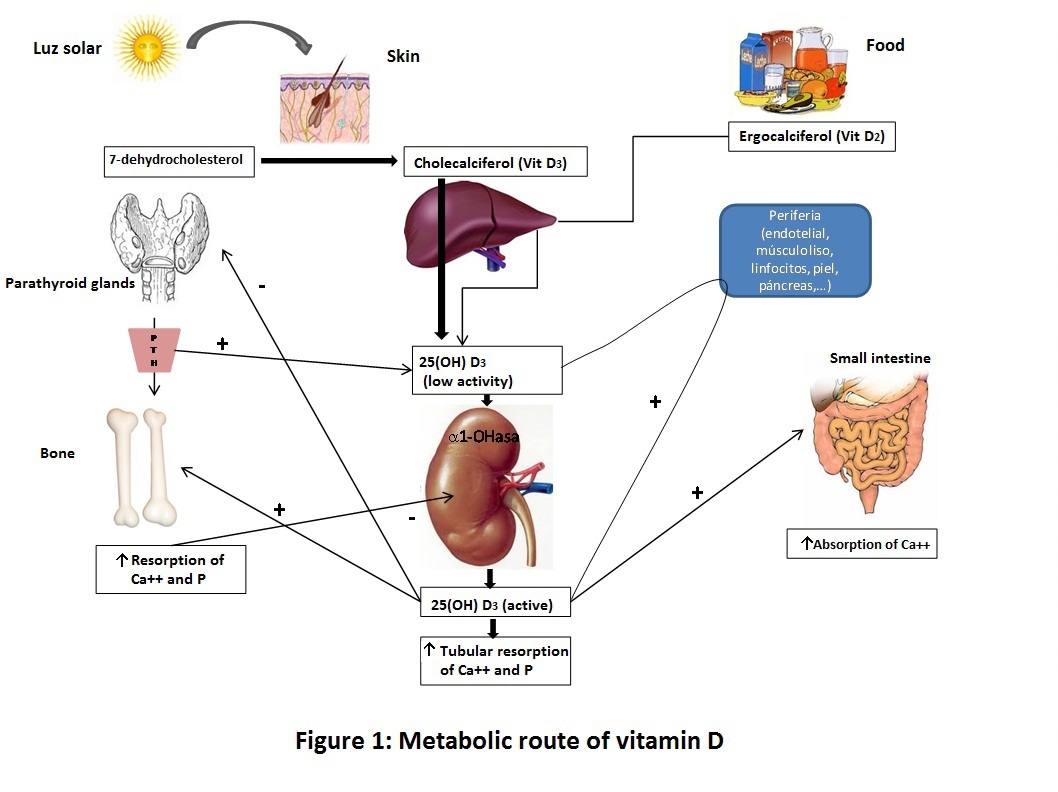
Different factors are involved in the metabolic pathway of vitamin D ranging from incorporation of vitamin D into the body until their arrival at the organs involved in homeostasis of calcium and phosphate, as well as the mechanisms by which VDR activation occurs in these organs (Figure 1).5,6
Vitamin D is more a hormone than an essential vitamin in the sense that it does not necessarily come from exogenous sources (foods) but can be produced from skin exposure to UVB radiation from sunlight. As a hormone, it travels through the circulatory system to distant organs and cells in order to coordinate their physiology and behavior, and is classically responsible for the regulation of calcium metabolism, promoting growth and proper remodeling of the bones. However, it is now known that vitamine D also has autocrine or paracrine effects in other extrarenal tissues such as skin, prostate, lymph nodes, intestine, breast, pancreas, spinal cord, brain, placenta and circulatory system, among others, where the 1α-hydroxylase enzyme is active (required for local production of calcitriol) or VDRs are also present (Figure 2).6 VDRs are ubiquitous and very abundant in the organs involved in calcium metabolism such as the intestines, increasing absorption of calcium and phosphate; the kidney which regulates reabsorption of calcium and phosphate, as well as the synthesis of calcitriol (1,25 (OH)2-vitamin D3 or 1,25D) through the parathyroid hormone (PTH) and the fibroblastic growth factor 23 (FGF-23)/Klotho complex; bone, where vitamin D is involved in the regulation of bone turnover and particularly in its adequate mineralization; and the parathyroid glands, on which vitamin D acts by inhibiting PTH synthesis and secretion. All biological actions, both of vitamin D and its synthetic analogs, are mediated via its binding to the VDR. In the different tissues, vitamin D and any of its metabolites may act in cases of disease or injury, as well as a compensatory mechanism.7 The other effects of VDR ligands, not related to calcium metabolism, are multiple, being their antiproliferative, differentiation-inducing and immunomodulatory effects the most significant. In fact, there are already VDR agonist drugs used for the treatment of psoriasis (calcipotriol, tacalcitol) with limited systemic absorption and use of ligands has been suggested for the treatment of inflammatory diseases (rheumatoid arthritis, psoriatic arthritis), dermatological diseases (in addition to psoriasis, also for photoageing), osteoporosis, breast or prostate cancer and autoimmune disorders.8
In the context of CKD, progressive loss of renal function leads to reduction of calcitriol and an impaired homeostasis of calcium, phosphate, PTH, FGF-23 and megaline, among others, which in turn have influence by different interactions on VDR activation, either directly or indirectly. When glomerular filtration rate (GFR) decreases, low calcitriol levels can be detected before elevation of PTH levels, so, together with phosphate restriction, it seems important to activate VDRs early in these patients, provided that it does not significantly alter phosphate control.9 In a cross-sectional study of an outpatient cohort conducted in 153 sites and 1,814 patients, 49% of patients had low calcitriol and high PTH levels, regardless of the levels of its precursor calcidiol (25 (OH)-vitamin D). 9 This study also confirmed the well-established close association between low calcitriol levels and prevalence of secondary hyperparathyroidism (SHP), so that SHP was present in this study in 56% of patients with a GFR10 <60 mL/min/1.73m2. However, it should also be taken into account that it is now well known that before a decrease in the levels of calcitriol occurs, an increase is observed in FGF-23, a phosphatonin also responsible for the reduction in 1-α-hydroxylase activity and increased activity of 24-hydroxylase. It is therefore important in early-stages of CKD to prevent retention of phosphate (before hyperphosphatemia develops).11,12 Moreover, the decrease in cofactor Klotho is another phenomenon of early CKD and Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) complex13 and it seems that the activation of VDRs could be potentially beneficial in terms of increasing its expression, as has been demonstrated in two experimental models.14,15
Currently all international guidelines recommend measuring calcidiol or 25-OH vitamin D levels (not calcitriol) in patients with CKD. Calcidiol levels represent the biochemical reflection of adequate exposure to and storage of vitamin D and the presence of vitamin D insufficiency. Vitamin D deficiency has been shown to be highly prevalent (>80%) in CKD patient. In addition, a close association has been reported between low calcidiol plasma levels (precursor of calcitriol) and cardiovascular morbidity, reported in subjects with CKD as well as in the general population. Plasma calcidiol is 100 to 1,000 times less potent than calcitriol, but its plasma concentrations are in a higher order of magnitude (ng/mL vs. pg/mL).6 However, it should be noted that although supplementation with native vitamin D (D3 or colecalciferol, D2 or ergocalciferol) may decrease PTH16 levels, this observation is not uniform in CKD patients.17 In Spain, oral calcidiol (calcifediol or Hidroferol®) is also widely used directly, although this requires caution in view of its activity and prolonged half-life.6 It is important to take into account that these derivatives are useful in supplementing or correcting vitamin D deficiencies (normalizing calcidiol levels). However, replacing calcidiol levels is not generally enough to correct SHP in the renal patient. In addition, its efficacy is much lower than active vitamin D (calcitriol, paricalcitol) or calcimimetics to reduce SHP in patients with CKD, but also, to date, an association between native vitamin D supplementation and the surrogate systemic pleiotropic benefits of cardiovascular disease has also been shown in one publication.18 However, no such association has ever been shown with overall or cardiovascular survival, not even in retrospective epidemiological cohort analyses. This is not so for active forms of vitamin D.19-21 Finally, though only in experimental models,22 it should be noted that it seems that combination therapies of active vitamin D (for instance, paricalcitol) and calcidiol, could increase the benefits of VDR activation; however, clinically the appropriate balance between these compounds to maximize the effects of activation and balance on degradation pathways (24-hydroxylase), is much less known and studied.
Based on these considerations, and beyond SHP and renal osteodystrophy, VDR activation together with phosphate restriction, among others, appear to play an important role in the treatment of CKD-MBD.9,23,24 The CKD-MBD complex confers a systemic nature to these disturbances that, in addition, occur early in the course of CKD.25,26 The systemic effects of VDR activation include their benefits on the cardiovascular tree and their close association with reduction of morbidity and mortality, as well as their potential actions on progression of CKD (Table 1).1,4,8,20,27
NONSELECTIVE AND SELECTIVE VDR ACTIVATORS
There are different types of vitamin D and so Table 2 shows a classification of the nomenclature used for the different types of vitamin D and VDR activators according to KDIGO and adapted in other studies.28
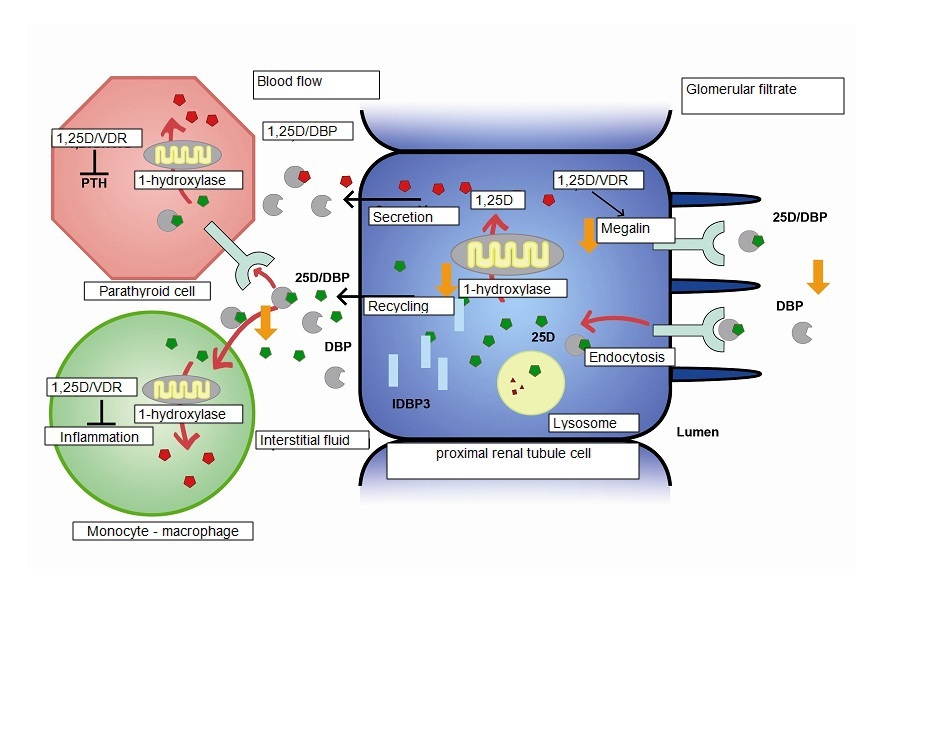
The VDR is a member of a nuclear receptor superfamily acting as a factor of ligand-dependent transcription of many genes related to synthesis and secretion of PTH and other proteins related to mineral metabolism, cell growth, and cell differentiation. A vitamin D receptor activator (VDRA) binds to VDR, and then transposes to the nucleus where it heterodimerizes with the retinoid X receptor (RXR). The resulting complex binds to the vitamin D responsive element (VDRE) in the promoter region of target (DNA) genes, recruiting transcription factors and co-regulatory molecules (transcription activators or inhibitors), acquiring the possibility of acting on multiple vitamin D target genes. For example, binding of the VDR/RXR complex to a negative VDRE in the PTH promoter gene suppresses its transcription (Figure 2).6,29 There are different VDRAs: In addition to the natural form of calcitriol, there are different synthetic analogs of vitamin D2 and D3, indicated for regulation of phosphate-calcium metabolism such as alfacalcidol, doxercalciferol, falecalcitriol, maxacalcitol, and paricalcitol. Calcitriol is the naturally active form of vitamin D and is approximately 500-1,000 times more active than its precursor 25-hydroxycholecalciferol.6,29 Paricalcitol and maxacalcitol are considered as selective VDR activators (sVDRAs).27,28
Regarding the difference between analogs and their effects on the different target organs is related, among other factors, to the affinity to circulating vitamin D binding protein (DBP). For instance, it has been shown that maxacalcitol has about 400 to 500 times less binding affinity to DBP than calcitriol30 and thus has a shorter half-life and is cleared more rapidly from the circulation. In addition, DBP decreases access of the analog to target tissues and thus helps prevent potential intoxication. Vitamin D is normally degraded by 24-hydroxylase (which is also induced by FGF-23), which carboxylates the carbon 24 in the side chains of the analogs, promoting their biological inactivation. Vitamin D analogs by binding to VDR in tissue, may remain longer in these and thus compromise vitamin D metabolism. It has also been shown that analogs have a lower affinity for VDR than calcitriol31 but differential regulation of 24-hydroxylase in target tissues determines the half-life of calcitriol and analogs32.
In addition, sVDRAs interact differentially with the above-mentioned cofactors (coactivators and/or cosuppressors) and, based on conformational differences occurring in these molecules, gene expression may be modified when this heterodimer binds to the VDRE of the promoter region of specific DNA, causing selective effects of DNA transcription in different cells and tissues. Calcitriol has ten times more affinity for binding to the VDR than the sVDRA paricalcitol27,31. However, this difference in binding affinity is not the same for all body tissues, essentially applying mainly to intestinal and bone tissues, as the affinity of paricalcitol for the VDR in the parathyroid glands is 3-4 times lower than that of calcitriol. Paricalcitol is less active than calcitriol in inducing homodimerization (VDR:VD) and heterodimerization of VDR: receptor-associated coactivator 3 (RAC3), and more active than calcitriol in inducing heterodimerization of VDR:RXR and VDR: glucocorticoid receptor interacting protein 1 (DRIP1).33
The interest in the pharmacological synthesis of sVDRAs such as paricalcitol and maxicalcitol appears as a result of the clinical need for expanding the therapeutic window of conventional forms of vitamin D and attempting to minimize the risk of hypercalcemia and hyperphosphatemia associated with the use of the nonselective derivatives calcitriol or alfacalcidol. sVDRAs allow synthesis and secretion of shp to be inhibited more efficiently and with a lower impact on intestinal absorption of calcium and phosphate. Therefore, they are attributed a lower risk of hypercalcemia, hyperphosphatemia, and elevated calcium-phosphate product levels, thereby avoiding possible effects derived from high levels of these metabolites in blood, including possible passive extraskeletal deposition of calcium and phosphate as vascular or valvular calcifications. Furthermore, these selective effects of sVDRA were also seen on gene expression in various types of cells and tissues, including the expression of molecules involved in the process of vascular calcification. Using DNA microarray technology to evaluate gene expression profiles in vascular smooth muscle cells incubated with calcitriol or paricalcitol, it was shown that, though most of the expression profile was similar, paricalcitol activates and deactivates different genes than calcitriol. These differences are not explained by differences in dose; thus, in an experimental model of active vascular calcification it was shown that paricalcitol, unlike calcitriol, does not increase the expression of transcription factor Cbfa1 (RunX2), which activates one of the signaling pathways for transformation of smooth muscle cells into bone-like cells.34 These observations have been studied further in vitro, demonstrating that paricalcitol prevents the activation of the phosphate-induced Wnt/β-catenin pathway and also reduces calcification, down-regulating the expression of BMP-2 and other osteoblast phenotype markers along with levels of β-catenin and its target genes.35,36
In other studies with microarrays in colon carcinoma cells, calcitriol and paricalcitol exhibit different profiles in gene regulation at different doses. Although different doses of
sVDRA may have been involved in such cases, differential gene expression suggests that differential effects of sVDRA may comprise, in part, the basis for the selectivity of sVDRA.6,29
In a 5/6 nephrectomized rat model, when paricalcitol is compared with calcitriol, its impact at the same doses is 3-4 times less than calcitriol on PTH levels and 10 times less on calcium and phosphate levels meaning that paricalcitol can act with a larger therapeutic margin for the prevention and treatment of SHP in early stages of CKD, as well as in patients on hemodialysis, and with a lower potential impact on vascular calcification.35,38 On the other hand, although there are no data on this action in humans, Malluche et al.39 state based on experimental data, that the vitamin D analogs paricalcitol and maxacalcitol could control PTH levels with a lower suppression of bone remodeling than that induced by calcimimetics.
In addition, sVDRA such as paricalcitol have been shown to be more effective than native vitamin D2 (ergocalciferol) in decreasing PTH levels in patients with stage 3 or 4 CKD with vitamin D deficiency and SHP.17 Although close associations have been shown of plasma calcidiol levels with overall and cardiovascular morbidity and mortality in both the general population and patients with CKD and/or hemodialysis, as well as the presence of some pleiotropic effects in patients on hemodialysis with use of cholecalciferol,18 as was mentioned above, there is no analysis showing an association between the use of native vitamin D (supplements) and improved survival.
COMPARATIVE STUDIES BETWEEN SELECTIVE AND NONSELECTIVE VDRAs AND CHANGES FROM NONSELECTIVE TO SELECTIVE VDRAs
As mentioned earlier, the primary objective of the development of sVDRAs is to reduce excess PTH and prevent hyperplasia of the parathyroid gland maintaining the beneficial effects of vitamin D and minimizing the undesirable effects on serum calcium and phosphate levels, as well as the potential induction of vascular calcification, particularly in the presence of elevated phosphate levels. Both in the 200940 KDIGO guidelines and current 2011 Spanish Society of Nephrology guidelines, it is considered reasonable to assess the presence/absence of vascular calcification to direct therapy of the CKD-MBD complex, due to its close association with mortality.41
Table 342-54 shows the existing comparative studies between sVDRAs, placebo, and/or other therapeutic options.
A recent randomized, prospective, crossover study compared alfacalcidol to intravenous paricalcitol in 80 patients on hemodialysis55 over a short period of 16 weeks. The proportion of patients achieving a 30% reduction in PTH levels in the last 4 weeks of treatment was 82% in the group treated with alfacalcidol and 93% in the group treated with paricalcitol. In addition, 18% and 31% of patients treated with alfacalcidol and paricalcitol, respectively, achieved the criterion for treatment success defined as PTH levels <300pg/mL, with levels of phosphate <1.8mmol/L and ionized calcium levels <1.3mmol/L. In no case did differences reach statistical significance, but it should be noted that, unfortunately, due to the period effect, there was no access to data on treatment crossover and only initial 16 week intervention period was analyzed for 80 of 117 patients estimated in the calculation of the sample size.
Finally, a recently published56 study analyzed the incremental cost-effectiveness ratio of paricalcitol versus alfacalcidol in a hypothetical cohort of patients with CKD. The authors concluded that paricalcitol offers short and long-term benefits in terms of health economics and the model used in the study suggests that the use of paricalcitol in patients with early CKD may be cost effective from the perspective of the British National Health Service (NHS) compared with the use of a nonselective VDR activator. There are two more economical evaluations, one in the United States where intravenous57 paricalcitol and calcitriol are compared and another in Germany58 where intravenous paricalcitol is compared to oral calcitriol and intravenous alfacalcidol. Both studies also concluded that paricalcitol has a better cost-effectiveness ratio.
Lastly, a study by Sprague et al.46 evaluated the safety and efficacy of intravenous paricalcitol and calcitriol in suppressing PTH levels in hemodialysis patients. From this study, it was found that treatment with paricalcitol reduces PTH levels faster (p=0.025) and with fewer sustained episodes of hypercalcemia and increased CaxP (p=0.008) than treatment with calcitriol.
COMPARATIVE STUDIES BETWEEN PARICALCITOL AND CALCIMIMETICS
Prospective randomized studies including calcimimetics in their design (CONTROL, TARGET, OPTIMA) compared standard therapy with any active vitamin D derivative and the inclusion of a calcimimetic, assessing its consequences. Table 4 shows the two directly comparative studies, with different strategies and algorithms, between paricalcitol and calcimimetics, called the ACHIEVE59 and IMPACT studies.60,61
The IMPACT study is a Phase IV multicenter, multinational, randomized clinical trial with a 28-week follow-up comparing treatment with IV paricalcitol (IV stratum) or oral paricalcitol (oral stratum) in monotherapy (rescue cinacalcet) versus cinacalcet plus low doses of vitamin D in patients receiving hemodialysis. The efficacy analysis showed that the proportion of patients achieving the objective of maintaining iPTH values between 150-300pg/mL in weeks 21-28 (assessment period) was higher in the paricalcitol group than in the cinacalcet+vitamin D group. The differences were statistically significant when the IV stratum or both strata combined were compared, but were not when the oral stratum was compared. The heterogeneity of the countries involved between those assigned to the oral or IV groups, in addition to potential differences between the oral and IV groups in the type of vitamin D used in combination with cinacalcet (oral alphacalcidol or IV doxercalciferol, respectively) may at least partly explain these differences. Moreover, FGF-23 levels increased with paricalcitol. Since the risk of mortality in dialysis patients seems to globally decrease with paricalcitol,19,63,64 the potential harmful effect of this increase in FGF-23 (associated with higher mortality rates) or the potential beneficial increase in Klotho mentioned previously are not fully understood.14 In any case, it seems advisable not to significantly alter the balance of calcium and phosphate in patients with CKD.
Furthermore, the preliminary pharmacoeconomic analysis of the IMPACT study revealed that the cost of treatment with paricalcitol was less than that of cinacalcet+vitamin D65 and that the costs of phosphate binders were similar in both treatment groups, so that the total cost (study drug+chelators) in the paricalcitol group was 41% lower than in the cinacalcet+vitamin D group.66
In another publication where the results of the ACHIEVE study are shown, the authors concluded that, in contrast to the secondary objectives, cinacalcet combined with vitamin D analogs was not more effective than vitamin D analogs in achieving the primary objective of the study (K/DOQI, PTH 150-300 and Ca x P < 55¸ 21% vs. 14%, p=0.231), primarily due to the fact that 19% of patients in the cinacalcet group had PTH levels below the normal range. It was, however, a more expensive treatment.67
When considering all these studies, it must be recognised that, although they are randomised, criticism could be made in terms of design: there were marked difficulties in recruitment, both in the screening phase and after the wash-out period; there were patient losses not estimated before hand, and either they did not know or they failed to analyse certain potentially important data such as the dialysate calcium concentration (defined as intervals).
The recently-published prospective EVOLVE study (EValuation Of cinacalcet therapy to Lower cardioVascular Events) is the most ambitious clinical trial ever conducted in this area68. This study randomized 3,883 patients with moderate-to-severe SHP to receive cinacalcet or placebo vs. standard therapy (vitamin D derivatives and/or phosphate binders). After a mean exposure of 21.2 months in the cinacalcet group vs. 17.5 months in the placebo group, cinacalcet did not significantly reduce the combined primary endpoint of mortality or major cardiovascular events (RR 0.93; 95% confidence interval 0.85-1.02; p=0.11) in the primary non-adjusted intention-to-treat analysis and by intention to treat. However, largely due to a significant loss of statistical power that resulted from the lower incidence of events than initially expected, forcing the study to be extended, we believe that we cannot consider this trial as negative, but rather as inconclusive. Moreover, there were significant biases in interpreting the results due to the high number of patients who discontinued cinacalcet in the treatment arm (due to the usual side effects of the drug) and those who received commercial brand cinacalcet in the theoretical placebo arm (up to 20% of patients).68 In the intention-to-treat analysis, by definition, the former were analyzed as if they had received cinacalcet for the whole duration of the study and the latter, as if they had never received cinacalcet. In contrast to the IMPACT study, in the EVOLVE study, the patients treated with cinacalcet had significantly lower levels of PTH, these differences being explained by the different characteristics of the patients included and the different therapeutic algorithms used.
The recommendations of the Spanish Society of Nephrology for managing mineral bone metabolism disorders in patients with CKD recall that alterations in serum levels of calcium, phosphate, PTH, alkaline phosphatase and vitamin D, among others, have all been associated with increased mortality in patients with CKD and/or hemodialysis, though there is still no definite proof of a causal relationship. Of course, the EVOLVE study does not allow us to deny the importance of controlling these parameters on the morbidity and mortality of patients on dialysis. Thus, clinical practice guidelines advise normalization of phosphate in all patients, provided reasonable measures are used and given the homogeneous and significant association with mortality of high phosphate levels in patients with CKD;41 in addition, some authors consider normalization of calcidiol levels in all stages. It is also thought that, aside from the use of VDRAs and sVDRAs (for which the presence or absence of vascular and/or valvular calcifications, tendency to hypercalcemia or hyperphosphatemia, may be determining factors when rationally choosing between both) for prevention and treatment of SHP, low doses of paricalcitol of 1-5µg/week should be maintained in patients on dialysis to ensure VDR activation,41 regardless of the hyperparathyroidism control goal. In fact, in a recent Italian publication,69 use of a VDRA was associated with improved survival in patients on dialysis, including PTH levels below 150pg/mL. This option should consider the risk, probably lower with paricalcitol) of inducing adynamic bone disease and thus promoting vascular calcifications, against the potential risk of increased mortality associated with the lack of activation of VDR caused by the suspension of ARVD in this context.
In general, although it has to be recognised that there are many recommendations and suggestions in this field which are not based on type 1A evidence (rare in Nephrology), it would seem reasonable to accept that all this information could be used to individualise decision making.
Conflict of interest
The authors declare the following potential conflicts of interest.
Dr. Jordi Bover has done conferences sponsored by Abbott, Amgen, Genzyme and Shire. He has also participated on the national and international advisory boards of Abbott, Amgen and Genzy.
Figure 1. Metabolic route of vitamin D
Figure 2. Renal mechanism of vitamin D receptor activation
11796_19115_39853_en_11796_table1.doc
Table 1. Skeletal and extraskeletal effects of vitamin D
11796_19115_39854_en_11796_table2.doc
Table 2. Nomenclature of vitamin D and VDR activators
11796_19115_39855_en_11796_table3.doc
Table 3. PTH suppression and effects on calcium and phosphate levels: paricalcitol vs. placebo and paricalcitol vs. calcitriolSupresión de hormona paratiroidea y efectos sobre los niveles de calcio y fosfato: paricalcitol frente a placebo y paricalcitol frente a c
11796_19115_39857_en_11796_table4.doc
Table 4. Comparative studies between paricalcitol and calcimimetics