The progression of chronic kidney disease (CKD) involves the development of alterations in mineral metabolism that are closely related to cardiovascular outcomes and bone disease. Hypomagnesemia is associated with more rapid progression of CKD and other comorbidities. Our objective was to analyze in CKD patients stages 3–4 the impact of the administration of magnesium (Mg) carbonate on bone mineral density (BMD) and hemodynamic changes associated with by vascular calcification (VC).
Material and methodsPatients with CKD stages 3–4 were randomized into controls (n=12) or intervention (n=7) group receiving 360mg of Mg carbonate daily during a 15-month period. Parameters related to mineral metabolism, BMD, VC, and pulse wave velocity (PWV) were evaluated.
ResultsSupplementation with Mg produced an increase in the urinary excretion of Mg while serum Mg levels remained stable and no episodes of hypermagnesemia were reported. In addition, no significant changes were found in the degree of VC assessed by Adragao index, however, both serum and urine Mg were significantly associated with a decrease in PWV, suggesting an increase in vascular compliance. Likewise, BMD did not change following treatment, but serum Mg significantly correlated with the levels of N-terminal propeptide of collagen alpha-1(I) chain (PINP), a marker of bone synthesis.
ConclusionsIn sum, these results suggest a possible beneficial effect of Mg on vascular compliance with no detrimental effects on bone status. In addition, our results highlight the need to consider monitorization of urinary Mg status in CKD patients.
La progresión de la enfermedad renal crónica (ERC) supone el desarrollo de alteraciones del metabolismo mineral que están estrechamente relacionadas con eventos cardiovasculares y enfermedad ósea. La hipomagnesemia se asocia con una progresión más rápida de la ERC, así como con otras comorbilidades. Nuestro objetivo fue analizar en pacientes con ERC estadios 3-4 el impacto de la administración de carbonato de magnesio (Mg) sobre la densidad mineral ósea (DMO) y los cambios hemodinámicos asociados a la calcificación vascular (CV).
Material y métodosLos pacientes con enfermedad renal crónica estadios 3-4 se distribuyeron aleatoriamente en los grupos control (n=12) o intervención (n=7), que recibió 360 mg de carbonato de Mg diariamente durante un periodo de 15 meses. Se evaluaron parámetros relacionados con el metabolismo mineral además de la DMO, CV, y velocidad de onda de pulso (VOP).
ResultadosLa suplementación con Mg produjo un aumento en la excreción urinaria de Mg, mientras que los niveles séricos de Mg permanecieron estables y no se reportaron episodios de hipermagnesemia. Además, no se observaron cambios significativos en cuanto al grado de CV valorado en base al índice de Adragao. No obstante, tanto los niveles séricos como urinarios de Mg se asociaron significativamente con un descenso en la VOP, lo que sugiere un aumento en la distensibilidad vascular. De manera similar, la DMO no se modificó con la administración del tratamiento, pero los niveles séricos de Mg correlacionaron significativamente con los del propétido N-terminal del colágeno tipo I (PINP), un marcador de la síntesis ósea.
ConclusionesEn conjunto, estos resultados sugieren un posible efecto beneficioso del Mg sobre la distensibilidad vascular sin efectos negativos a nivel óseo. Además, nuestros resultados subrayan la necesidad de considerar la monitorización del nivel de Mg urinario en pacientes con ERC.
Chronic kidney disease (CKD) is a major health problem worldwide.1 It is estimated that more than 10% of the world population suffers from CKD2 and it is expected to become one of the leading causes of death in the next decades.3 The progressive loss of renal function leads to a variety of complications affecting the cardiovascular system, bone health, inflammatory and metabolic regulation, and others. Renal dysfunction causes abnormalities in phosphate (P) and calcium metabolism.4 The abnormalities in calcium-phosphate regulation are largely responsible for the development of vascular calcifications and the increase in vascular stiffness in CKD,5 which in turn causes an increase in pulse wave velocity (PWV), an independent predictor of cardiovascular mortality in CKD.6
Experimental work7,8 has shown that magnesium (Mg) supplementation reduces vascular calcifications (VC). In CKD, hypomagnesemia is associated with cardiovascular and other comorbidities that have been summarized by Rodelo-Haad et al.9 In CKD patients, lower levels of Mg are associated with a two-fold increased risk of progression to end-stage renal disease.10 Moreover, in patients on renal replacement therapy, a better survival rate is observed in those patients with slightly high Mg levels.10 However, there are no clinical studies to substantiate the potential beneficial effect of Mg supplementation on vascular stiffness as assessed by PWV.
The effect of Mg on bone has been a matter of discussion. Clinical studies have suggested an association between oral Mg and fractures,11 yet Mg may promote osteogenesis by the activation of Notch signaling.12 Altogether, evidence suggests that Mg administration may have benefits in patients with CKD.
Hence, our objective was to analyze in CKD patients stages 3–4 the impact of the administration of Mg supplements in clinical aspects of renal disease such as vascular compliance as measured by PWV, VC, and parameters of mineral and bone health.
MethodsStudy protocolWe performed a randomized, open-label, parallel-group clinical trial called MagicalBone. All subjects signed the informed consent for inclusion in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Cordoba (Cordoba Research Ethics Committee, Spain. Record number: 280 and 287, Committee file number: 3953).
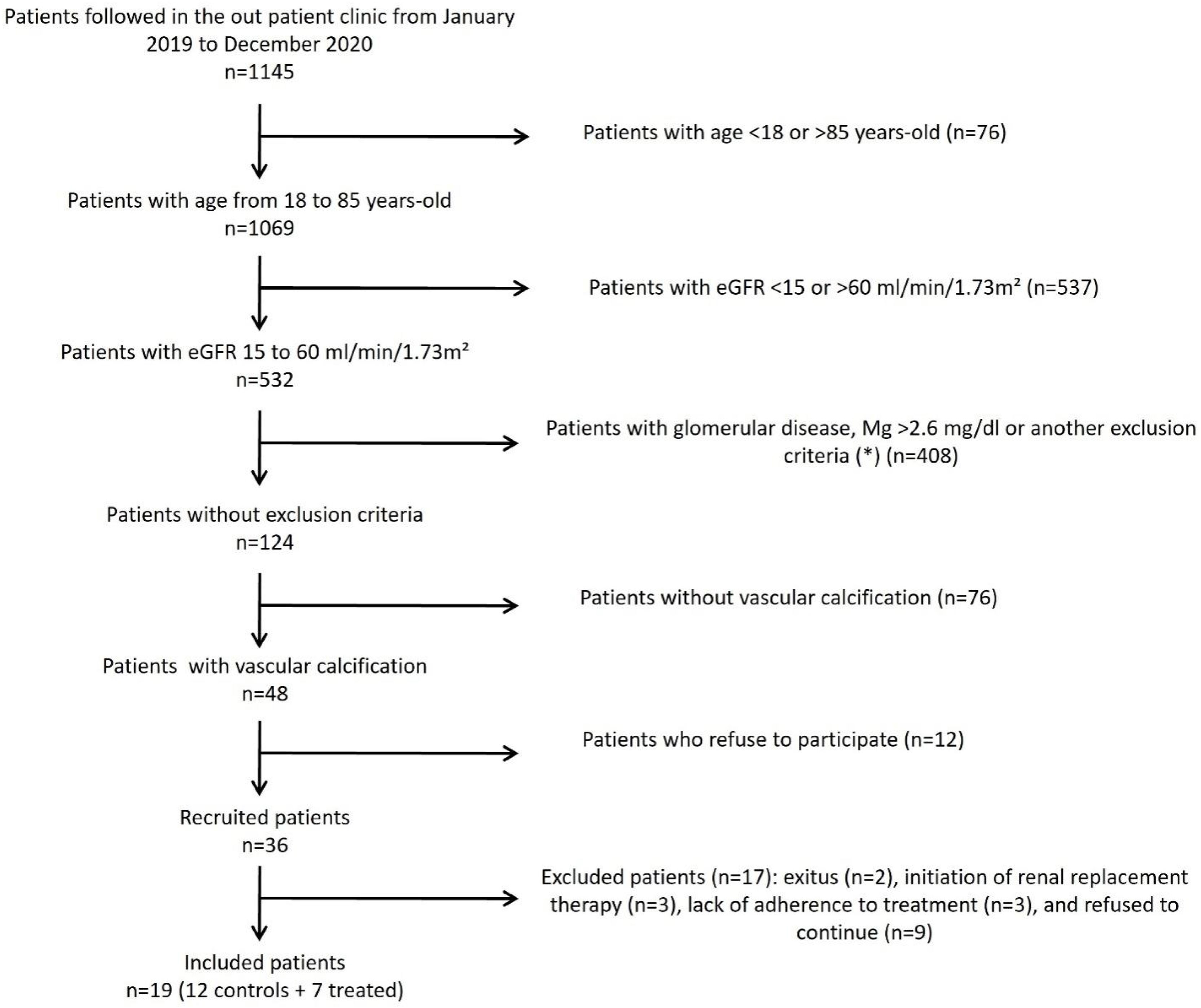
Thirty-six patients were recruited, and nineteen subjects completed the 15-month follow-up period. These patients were distributed into the control (n=12) and intervention (n=7) groups. Inclusion criteria were an estimated glomerular filtration rate (eGFR) between 15 and 60ml/min/1.73m2 (CKD stages 3–4), which was stable during the three months prior to randomization, and evidence of VC assessed by X-ray. Exclusion criteria were as follows: serum Mg concentration above 2.6mg/dl, glomerular disease, calciphylaxis, immediate need for renal replacement therapy, parathyroidectomy, treatment with biphosphonates or denosumab, HIV infection, hepatitis B or C, chronic liver disease, systemic inflammatory disease, recombinant or immunosuppressive therapies, or previous history of cancer in the last 5 years.
The participant flow chart is shown in Fig. 1. All patients were followed in the outpatient clinic by the same physician at least twice yearly before being included in the study. Baseline data included age, gender, body mass index, blood pressure, and comorbidities, or prevalent diseases. Patients were randomized into a control group and an intervention group that received 360mg of powdered Mg carbonate per day (Ana María Lajusticia®, Distribuciones Feliu S.L., Barcelona, Spain) during 15±1.5 months.
Flow chart showing the inclusion of the participants in the study. eGFR: estimated glomerular filtration rate, Mg: magnesium. (*) Exclusion criteria: calciphylaxis, immediate need for renal replacement therapy, parathyroidectomy, treatment with bisphosphonates or denosumab, HIV infection, hepatitis B or C, chronic liver disease, systemic inflammatory disease, recombinant or immunosuppressive therapies, or previous history of cancer in the last 5 years were excluded.
Blood was collected for measurement of standard plasma biochemistry and complete blood count. Twenty-four-hour urine samples were collected for quantification of Mg and Cr using an Architect c-16000 (Abbott®, Chicago, Illinois, USA). The eGFR was calculated by the CKD-EPI formula.13 Serum Mg was quantified in the local clinical analysis laboratory (reference values: 1.7–2.7mg/dl). Plasma P was determined by spectrophotometry (Biosystems, Barcelona, Spain). ELISA assays were used for the measurement of plasma intact FGF23 (iFGF23, Kainos, Japan), PTH (Quidel Corporation), the Wnt/β-catenin pathway inhibitors secreted frizzled-related protein 1 (SFRP1) (Cusabio, Wuhan, China) and Dickkopf protein 1 (DKK1) (Merck Millipore, Darmstadt, Germany), and the markers of bone formation N-terminal propeptide of collagen alpha-1(I) chain (PINP) (FineTest Biotech Inc., Wuhan, China), and bone resorption C-terminal telopeptides of Type I collagen (β-CTX and α-CTX for urine and plasma, respectively) (Immunodiagnostic Systems Holdings Ltd., Boldon, United Kingdom).
Assessment of vascular calcification, pulse wave velocity, and bone mineral densityAt the beginning and at the end of the study several tests were performed. The PWV was determined with a Mobil-O-Graph device (IEM GmbH, Stolberg, Germany). X-ray of pelvis and both hands was performed to assess and score the presence of VC according to Adragao index. Bone mineral density (BMD) in column, hip, and femoral neck was determined by densitometry (DXA: trademark LUNA, model DPX-NT FULLSIZE).
Statistical analysisContinuous variables are shown as mean±standard deviation (SD) or median (interquartile range, IQR). Categorical variables are presented as a percent (%). Statistical differences between both control and intervention groups were assessed by t-test, or the corresponding no parametric test. Univariate correlation analysis (Spearman) was used to identify the relationship between urine and plasma magnesium with other variables. A P-value inferior to 0.05 was considered statistically significant. Statistical analyses were performed using SPSS statistical program (SPSS Inc., Chicago, IL, USA).
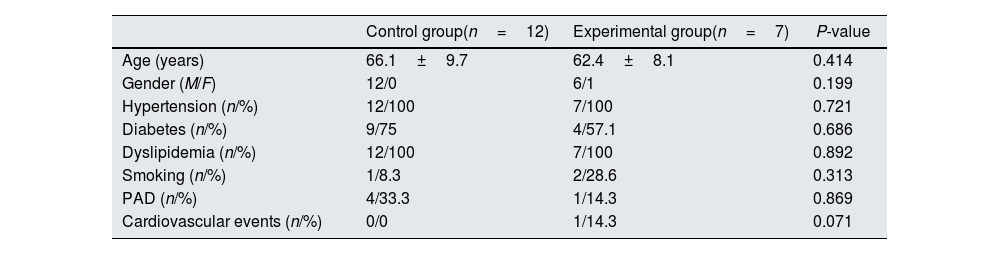
ResultsCharacteristics of the study population at baselineThe subjects who completed the follow-up period were randomized into the control (n=12) and the intervention (n=7) groups. Demographical and clinical characteristics of the study population are shown in Table 1. Age and gender were similar in both groups (P=0.414 and 0.199, respectively). In addition, the proportion of patients with hypertension (P=0.721), diabetes (P=0.686), dyslipidemia (P=0.892), and smoking (P=0.313) was also similar and no statistical differences were observed in the occurrence of peripheral arterial disease (PAD) (P=0.869) or other cardiovascular events (P=0.071).
Demographical and clinical characteristics of the patients included in the study.
| Control group(n=12) | Experimental group(n=7) | P-value | |
|---|---|---|---|
| Age (years) | 66.1±9.7 | 62.4±8.1 | 0.414 |
| Gender (M/F) | 12/0 | 6/1 | 0.199 |
| Hypertension (n/%) | 12/100 | 7/100 | 0.721 |
| Diabetes (n/%) | 9/75 | 4/57.1 | 0.686 |
| Dyslipidemia (n/%) | 12/100 | 7/100 | 0.892 |
| Smoking (n/%) | 1/8.3 | 2/28.6 | 0.313 |
| PAD (n/%) | 4/33.3 | 1/14.3 | 0.869 |
| Cardiovascular events (n/%) | 0/0 | 1/14.3 | 0.071 |
PAD: peripheral arterial disease.
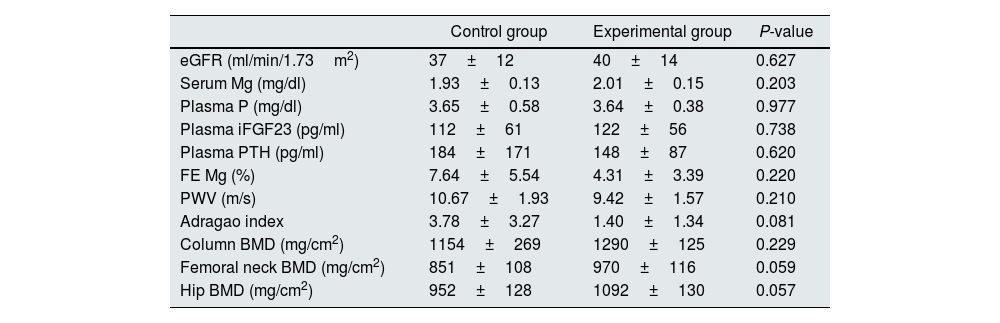
As shown in Table 2, at baseline control and intervention groups had similar values of eGFR (37±12 vs. 40±14ml/min/1.73m2, P=0.627), serum levels of Mg (1.93±0.13 vs. 2.01±0.15mg/dl, P=0.203), P (3.65±0.58 vs. 3.64±0.38mg/dl, P=0.977), iFGF23 (112±61 vs. 122±56pg/ml, P=0.738), and PTH levels (184±171 vs. 148±87pg/ml, P=0.620). In addition, the urinary excretion of Mg expressed as fractional excretion (FE) was similar in both arms (7.64±5.54 vs. 4.31±3.39%, respectively, P=0.220). Finally, no differences were found at the beginning of the study in PWV (10.67±1.93 vs. 9.42±1.57m/s, P=0.210), VC calculated by Adragao index (3.78±3.27 vs. 1.40±1.34, P=0.081) or BMD at column (1154±269 vs. 1290±125mg/cm2, P=0.229), femoral neck (851±108 vs. 970±116mg/cm2, P=0.059), and hip (952±128 vs. 1092±130mg/cm2, P=0.057) level (Table 2).
Comparison of the main variables in both arms at baseline.
| Control group | Experimental group | P-value | |
|---|---|---|---|
| eGFR (ml/min/1.73m2) | 37±12 | 40±14 | 0.627 |
| Serum Mg (mg/dl) | 1.93±0.13 | 2.01±0.15 | 0.203 |
| Plasma P (mg/dl) | 3.65±0.58 | 3.64±0.38 | 0.977 |
| Plasma iFGF23 (pg/ml) | 112±61 | 122±56 | 0.738 |
| Plasma PTH (pg/ml) | 184±171 | 148±87 | 0.620 |
| FE Mg (%) | 7.64±5.54 | 4.31±3.39 | 0.220 |
| PWV (m/s) | 10.67±1.93 | 9.42±1.57 | 0.210 |
| Adragao index | 3.78±3.27 | 1.40±1.34 | 0.081 |
| Column BMD (mg/cm2) | 1154±269 | 1290±125 | 0.229 |
| Femoral neck BMD (mg/cm2) | 851±108 | 970±116 | 0.059 |
| Hip BMD (mg/cm2) | 952±128 | 1092±130 | 0.057 |
Data are expressed as mean±standard deviation.
eGFR: estimated glomerular filtration rate based on CKD-EPI equation, Mg: magnesium, P: phosphorus, iFGF23: intact FGF23, PTH: parathyroid hormone, FE: fractional excretion, PWV: pulse wave velocity, BMD: bone mineral density.
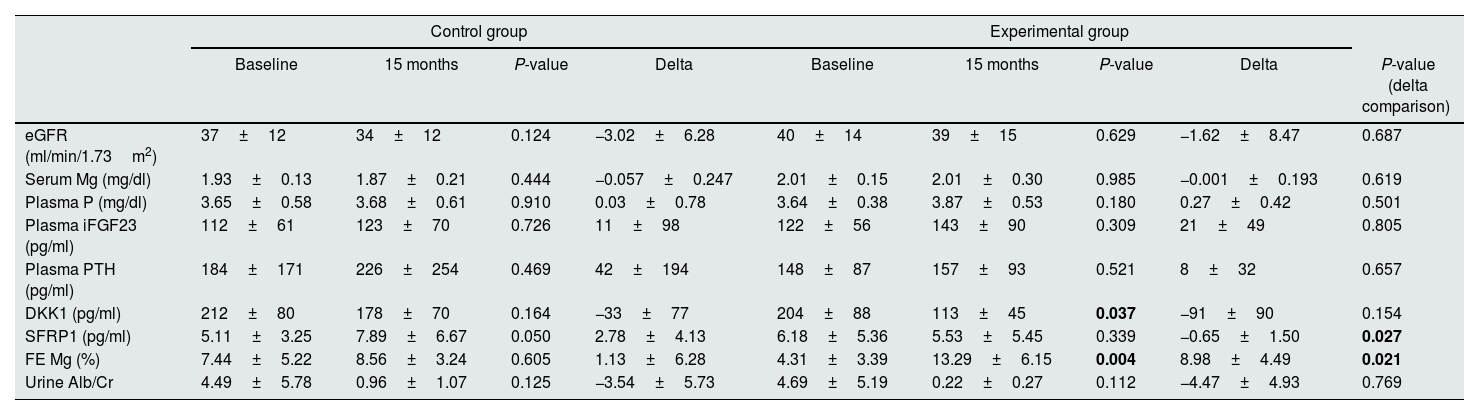
The comparison between the values of the variables studied at baseline and after 15 months in control and intervention groups is shown in Table 3. The eGFR did not change significantly in either group; however, although it did not reach statistical significance, the rate of CKD progression (change in eGFR) had a tendency to be slower in the Mg-treated group (−1.62±8.47 vs. −3.02±6.28ml/min/1.73m2 in the control group, P=0.687).
Comparison of the variables related to CKD and mineral metabolism at the beginning and at the end of the study. In addition, the comparison between the magnitude of the changes found in the control and the experimental group is also shown.
| Control group | Experimental group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 15 months | P-value | Delta | Baseline | 15 months | P-value | Delta | P-value (delta comparison) | |
| eGFR (ml/min/1.73m2) | 37±12 | 34±12 | 0.124 | −3.02±6.28 | 40±14 | 39±15 | 0.629 | −1.62±8.47 | 0.687 |
| Serum Mg (mg/dl) | 1.93±0.13 | 1.87±0.21 | 0.444 | −0.057±0.247 | 2.01±0.15 | 2.01±0.30 | 0.985 | −0.001±0.193 | 0.619 |
| Plasma P (mg/dl) | 3.65±0.58 | 3.68±0.61 | 0.910 | 0.03±0.78 | 3.64±0.38 | 3.87±0.53 | 0.180 | 0.27±0.42 | 0.501 |
| Plasma iFGF23 (pg/ml) | 112±61 | 123±70 | 0.726 | 11±98 | 122±56 | 143±90 | 0.309 | 21±49 | 0.805 |
| Plasma PTH (pg/ml) | 184±171 | 226±254 | 0.469 | 42±194 | 148±87 | 157±93 | 0.521 | 8±32 | 0.657 |
| DKK1 (pg/ml) | 212±80 | 178±70 | 0.164 | −33±77 | 204±88 | 113±45 | 0.037 | −91±90 | 0.154 |
| SFRP1 (pg/ml) | 5.11±3.25 | 7.89±6.67 | 0.050 | 2.78±4.13 | 6.18±5.36 | 5.53±5.45 | 0.339 | −0.65±1.50 | 0.027 |
| FE Mg (%) | 7.44±5.22 | 8.56±3.24 | 0.605 | 1.13±6.28 | 4.31±3.39 | 13.29±6.15 | 0.004 | 8.98±4.49 | 0.021 |
| Urine Alb/Cr | 4.49±5.78 | 0.96±1.07 | 0.125 | −3.54±5.73 | 4.69±5.19 | 0.22±0.27 | 0.112 | −4.47±4.93 | 0.769 |
Data are expressed as mean±standard deviation.
eGFR: estimated glomerular filtration rate based on CKD-EPI equation, Mg: magnesium, P: phosphorus, iFGF23: intact FGF23, PTH: parathyroid hormone, DKK1: dickkopf protein 1, SFRP1: secreted frizzled related protein 1, FE: fractional excretion, Alb: albumin, Cr: creatinine.
The change in serum Mg levels was not different between the two groups (−0.057±0.247 vs. −0.001±0.193mg/dl in the control and the Mg-treated group, respectively, P=0.619). In this regard, patients did not report adverse effects related to hypermagnesemia. Two out of the excluded patients were using Mg supplements and reported side effects, leading to their exclusion due to self-limited diarrheal stools and nonspecific epigastric pain. Interestingly, in the intervention group, after 15 months of treatment serum Mg concentration significantly correlated negatively with PWV (R2=−0.986, P<0.001) and positively with circulating DKK1 (R2=0.775, P=0.041) (Table 4).
Significant correlation analysis found among variables collected from the study population.
| Control group | Experimental group | Control group | Experimental group | ||
|---|---|---|---|---|---|
| Mg-PWV | R2=−0.407P=0.317 | R2=−0.986P<0.001 | FE Mg-PWV | R2=0.086P=0.872 | R2=−0.900P=0.037 |
| Mg-DKK1 | R2=0.329P=0.296 | R2=0.775P=0.041 | FE Mg-PINP | R2=−0.017P=0.966 | R2=0.899P=0.015 |
Mg: magnesium, PWV: pulse wave velocity, DKK1: Dickkopf protein 1, FE: fractional excretion, PINP: N-terminal propeptide of collagen alpha-1(I) chain.
The administration of oral Mg produced a significant increase in the FE of Mg in the intervention group as compared to controls (1.13±6.28 vs. 8.98±4.49%, P=0.021) (Table 3). In addition, the FE of Mg was also significantly correlated with the PWV (R2=−0.900, P=0.037) as well as with the circulating levels of PINP (R2=0.899, P=0.015) (Table 4).
No difference was observed between the controls and the Mg-treated patients in the delta of change of plasma P (0.03±0.78 vs. 0.27±0.42mg/dl, P=0.501), iFGF23 (11±98 vs. 21±49pg/ml, P=0.805), PTH (42±194 vs. 8±32pg/ml, P=0.657), and DKK1 levels (−33±77 vs. −91±90pg/ml, P=0.154), although the levels of circulating DKK1 were significantly reduced in the intervention arm at the end of the study (204±88 vs. 113±45pg/ml, P=0.037) (Table 3). When analyzed the change in plasma SFRP1 for each group, we also observed a significant reduction in the Mg-treated arm (2.78±4.13 vs. −0.65±1.50pg/ml, P=0.027) (Table 3).
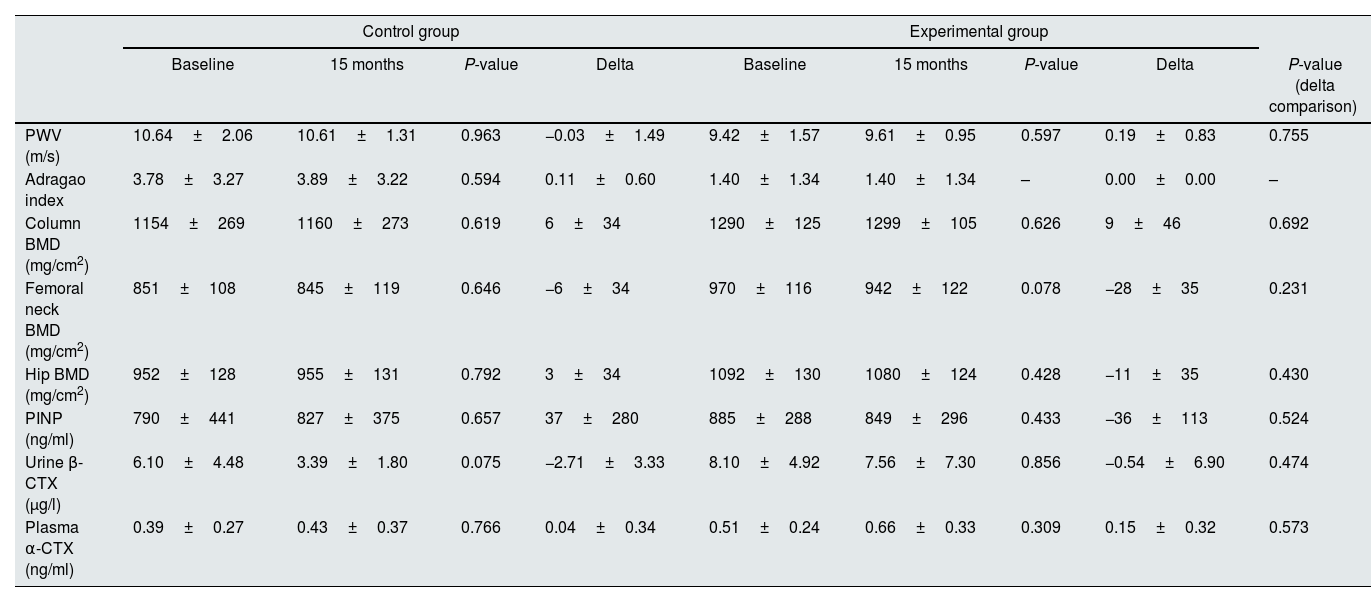
Effect of Mg supplementation on hemodynamic parameters, progression of VC, and bone statusAt the end of the study, the PWV was similar in the control and the intervention group (−0.03±1.49 vs. 0.19±0.83m/s, respectively, P=0.755) (Table 5). However, as previously mentioned, the PWV at the end of the follow-up was significantly correlated with serum Mg and FE of Mg in urine only in the intervention group (Table 4).
Comparison of the changes in hemodynamic parameters, progression of vascular calcification, and bone status at the beginning and at the end of the study for each experimental group.
| Control group | Experimental group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 15 months | P-value | Delta | Baseline | 15 months | P-value | Delta | P-value (delta comparison) | |
| PWV (m/s) | 10.64±2.06 | 10.61±1.31 | 0.963 | −0.03±1.49 | 9.42±1.57 | 9.61±0.95 | 0.597 | 0.19±0.83 | 0.755 |
| Adragao index | 3.78±3.27 | 3.89±3.22 | 0.594 | 0.11±0.60 | 1.40±1.34 | 1.40±1.34 | – | 0.00±0.00 | – |
| Column BMD (mg/cm2) | 1154±269 | 1160±273 | 0.619 | 6±34 | 1290±125 | 1299±105 | 0.626 | 9±46 | 0.692 |
| Femoral neck BMD (mg/cm2) | 851±108 | 845±119 | 0.646 | −6±34 | 970±116 | 942±122 | 0.078 | −28±35 | 0.231 |
| Hip BMD (mg/cm2) | 952±128 | 955±131 | 0.792 | 3±34 | 1092±130 | 1080±124 | 0.428 | −11±35 | 0.430 |
| PINP (ng/ml) | 790±441 | 827±375 | 0.657 | 37±280 | 885±288 | 849±296 | 0.433 | −36±113 | 0.524 |
| Urine β-CTX (μg/l) | 6.10±4.48 | 3.39±1.80 | 0.075 | −2.71±3.33 | 8.10±4.92 | 7.56±7.30 | 0.856 | −0.54±6.90 | 0.474 |
| Plasma α-CTX (ng/ml) | 0.39±0.27 | 0.43±0.37 | 0.766 | 0.04±0.34 | 0.51±0.24 | 0.66±0.33 | 0.309 | 0.15±0.32 | 0.573 |
The comparison between the magnitude of the changes found in the normal and the experimental group is also shown. Data expressed as mean±standard deviation.
PWV: pulse wave velocity, BMD: bone mineral density, PINP: N-terminal propeptide of collagen alpha-1(I) chain, b-CTX: urine C-terminal telopeptides of Type I collagen, a-CTX: plasma C-terminal telopeptides of Type I collagen.
The change in VC scored according to Adragao index was similar in both groups (Table 5), although the score slightly increased without reaching statistical signification in the control group (0.11±0.60) and remained unchanged in the patients receiving Mg.
Bone mineral density assessed in column, hip, and femoral neck, did not change significantly in both control and intervention groups (6±34 vs. 9±46mg/cm2, P=0.692; −6±34 vs. −28±35mg/cm2, P=0.231; and 3±34 vs. −11±35mg/cm2, P=0.430, respectively) (Table 5). At the end of the follow-up, the levels of the telopeptides PINP, marker of bone synthesis, and plasma α-CTX and urine β-CTX, indicators of bone resorption, did not change following the administration of Mg, being the respective delta of change 37±280 vs. −36±113ng/ml (P=0.524), −2.71±3.33 vs. −0.54±6.90μg/l (P=0.474), and 0.04±0.34 vs. 0.15±0.32ng/ml (P=0.573) (Table 5).
DiscussionThis study has evaluated the effect of the administration of a supplement of Mg for 15 months to patients with CKD stages 3–4 and VC. In particular, CKD progression, bone status, and parameters related to mineral metabolism and VC have been analyzed. The adherence to treatment was suggested by a significant increase in the urinary excretion of Mg in patients receiving Mg supplements; however, serum levels of Mg remained stable. Estimated glomerular filtration rate was not modified by the administration of Mg, as well as bone mineral density and bone markers of bone resorption or bone formation. Vascular calcification, assessed by X-rays, did not improve after Mg supplementation. Nevertheless, in patients receiving Mg, the serum level of Mg and also the FE Mg were inversely correlated with PWV, suggesting an improvement in vascular compliance. Interestingly, strong associations between FE Mg and PINP levels and PWV were also observed. Neither BMD nor VC were modified in the group of patients receiving oral Mg for 15 months.
Patients were treated daily with 360mg Mg carbonate (28.7% of elemental Mg). The administration of Mg carbonate, alone or in combination with calcium carbonate, has been already associated with no increase or, at least, not symptomatic increase in serum Mg levels.14–17 In our study, serum Mg levels remained unchanged in the treated group, with no reported undesirable side effects.
Despite no changes in serum Mg levels, we found that the Mg-treated arm exhibited a marked increase in the urinary excretion of Mg, expressed as FE Mg. Altogether, the results observed in both serum and urine Mg in the intervention group might be interpreted as the result of the replenishment of the intracellular Mg stores following supplementation, with the excess of Mg being eliminated by urine excretion. This supports the notion of the usefulness of urine as a source to estimate Mg status, as it has been recently reported.18 In this regard, it is important to consider whether Mg serum levels might accurately represent the body Mg content. As it has been previously published,19 total serum Mg might not precisely reflect the actual Mg availability. This is due to the fact that ionized Mg is the biologically active form and it may be affected by factors such as pH, the presence of other ligands, or even alterations in the stability of the sample.20 In addition, it has been reported that less than 1% of total Mg is found in serum.21 This fact might be particularly relevant in CKD where, despite showing hypermagnesemia due to low filtration induced Mg retention, intracellular Mg stores may not be repleted.9
One of the main goals of this work was to assess bone status following the administration of a Mg supplement. Bone mineral density was evaluated at three levels: column, femoral neck, and hip. The effect of Mg in bone has not been totally clarified so far and it has been suggested a relationship between Mg and osteoporosis.22 However, Spiegel et al. did not find significant changes in vertebral BMD in dialysis patients following the administration of Mg carbonate/Ca carbonate for 18 months.23 More recently, two systematic reviews and meta-analyses concluded that a higher Mg intake is associated with increased hip and femoral neck BMD.24,25 In our study, we did not find changes in BMD at the three locations evaluated. However, we determined the levels of PINP, a marker of bone formation,26 and although circulating PINP did not change after the follow-up, it showed a strong and positive correlation with urine Mg excretion. In this regard, it should be noted that the measurement of total PINP may be affected since monomeric PINP accumulates in CKD.27 However, this limitation was overcome in our study given that no changes in the GFR occurred throughout the study.
Furthermore, plasma levels of DKK1 were significantly reduced in those patients receiving oral Mg at the end of the study. The role of DKK1 in bone metabolism has been already studied. In fact, deletion of DKK1 is associated with an increase in bone formation,28 whereas DKK1 overexpression induces osteopenia.29 Thus, it could be hypothesized that Mg supplementation might induce an activation of Wnt/β-catenin pathway by reducing the Wnt activators DKK1 and SFRP1, thus contributing to better bone health. This notion is supported by the findings reported by us, showing that Mg acts as an osteoinductor in vitro, promoting the differentiation of mesenchymal stem cells into osteoblasts in a process that is mediated by Notch signaling.12 However, this effect seems to be dual depending on Mg concentration.30 Although it could not be ruled out that the bone response to Mg is also altered under uremic conditions, we have observed that the administration of high dietary Mg improved bone parameters in CKD rats, suggesting an osteoblastogenic effect similar to that found in vitro.8 Nonetheless, more studies are needed to clarify the mechanisms involved in this effect.
The development and progression of VC is one of the most frequent, complex, and serious complications in CKD. Calcification was assessed in our study by calculating the Adragao index in X-ray images of hands and pelvis. The impact of Mg administration on the development and progression of VC has been studied in both clinical and experimental approaches. In uremic animals, several works have repeatedly reported the effect that Mg exerts preventing and even reversing VC.8,31 Different physicochemical and cell-mediated mechanisms have been proposed to mediate this effect,32 but additional effects as an inhibitor of inflammation and oxidative stress at vascular level have been recently suggested.33 Clinical studies have shown non-uniform results. In two quite recent works, Sakaguchi and cols. found that oral Mg administration slowed down coronary arterial calcification, yet Bressendorf et al. failed to find any change in this parameter. Such differences may be attributable to methodological differences in the MAGiCAL-CKD trial.34,35 In our work, we did not detect any differences in VC between the control and the Mg-treated group, although the change over time in the intervention group showed a trend to be lower. Magnesium oxide was administered to patients in the work carried out by Sakaguchi, containing a substantially higher amount of elemental Mg (198mg) when compared with the Mg compound used in our study. In line with this effect at vascular level, we found an interesting association between serum and urine Mg and PWV, indicator of arterial stiffness. In this regard, several studies performed following different experimental approaches have reported variable results.36–38 Experimental studies have demonstrated that the administration of dietary Mg to uremic rats is associated with lower oxidative stress and reduced expression of inflammatory markers at vascular level, along with a better hemodynamic profile.33 Altogether, these results allow to hypothesize a possible direct effect of Mg on the vascular wall.
We must admit some limitations in our study. First, the study population comprised a low number of patients because of the strict inclusion criteria and the fact that some patients did not complete the study. In particular, some patients were excluded from the experimental group due to lack of adherence to treatment, which might have been due to the side effects attributed to the administration of low doses of Mg, such as abdominal pain and diarrheal stools. Second, although unintentionally, the number of women was limited in our study, which may lead to a gender bias. However, the dose of Mg administered covers the requirements for both male and female and therefore, similar effects derived from Mg supplementation might be expected in both genders. Third, the sum of the low sensitivity for longitudinal changes with the limited sample could be related to the extreme values that some patients had in the control group. Fourth, the follow-up was 15 months, period that may be considered relatively short compared with other studies. Finally, the Mg compound administered may contain lower elemental or bioavailable Mg in comparison with other molecules. Undoubtedly, these factors alone or in combination might have contributed to underestimate the effect of Mg on the parameters studied due to methodological aspects or lack of statistical power.
ConclusionsIn sum, the results obtained here add information on the effects of Mg in parameters such as vascular stiffness, a key factor in the progression of CKD, and to the occurrence of undesirable cardiovascular events. Thus, it might be considered monitoring serum Mg levels and urinary excretion of Mg. Nevertheless, more studies are needed to fully understand the role of this element in the pathophysiology of CKD and its complications.
FundingThis work has been funded by the Spanish Society of Nephrology (Ayuda a la Investigación en Nefrología, Año 2018), the Programa Nacional de I+D+I 2013-2016 co-financed with European Funds (FEDER) from the Spanish Government (grants PI17/01010, PI18/00138, PI20/0660, and PI20/01645), Consejeria de Salud de la Junta de Andalucia (PI-0154-2017 and PI-0071-2021), Consejería de Transformación Económica, Industria y Conocimiento (PY20_00773), RICORS2040, and European Uremic Toxin Work Group (EUTox).
Conflict of interestAuthors have no conflicts of interest to declare.
M.V.P.-R.M. is a recipient of a Juan Rodés Contract from Carlos III Health Institute (2021/2025). M.E.R.-O. is supported by the Miguel Servet Program from Carlos III Health Institute (ISCIII) (CP21/00048). J.R.M.-C. is a senior researcher supported by the Nicolás Monardes Programme, Consejería de Salud-Servicio Andaluz de Salud (Junta de Andalucía). We are grateful to Ana María Lajusticia for providing Mg carbonate to carry out the study. All authors have read and approved the final manuscript.