Historically, it takes an average of 17 years to move new treatments from clinical evidence to daily practice. Given the highly effective treatments now available to prevent or delay kidney disease onset and progression, this is far too long. The time is now to narrow the gap between what we know and what we do. Clear guidelines exist for the prevention and management of common risk factors for kidney disease, such as hypertension and diabetes, but only a fraction of people with these conditions worldwide are diagnosed, and even fewer are treated to target. Similarly, the vast majority of people living with kidney disease are unaware of their condition, because in the early stages it is often silent. Even among patients who have been diagnosed, many do not receive appropriate treatment for kidney disease. Considering the serious consequences of kidney disease progression, kidney failure, or death, it is imperative that treatments are initiated early and appropriately. Opportunities to diagnose and treat kidney disease early must be maximized beginning at the primary care level. Many systematic barriers exist, ranging from patient to clinician to health systems to societal factors. To preserve and improve kidney health for everyone everywhere, each of these barriers must be acknowledged so that sustainable solutions are developed and implemented without further delay.
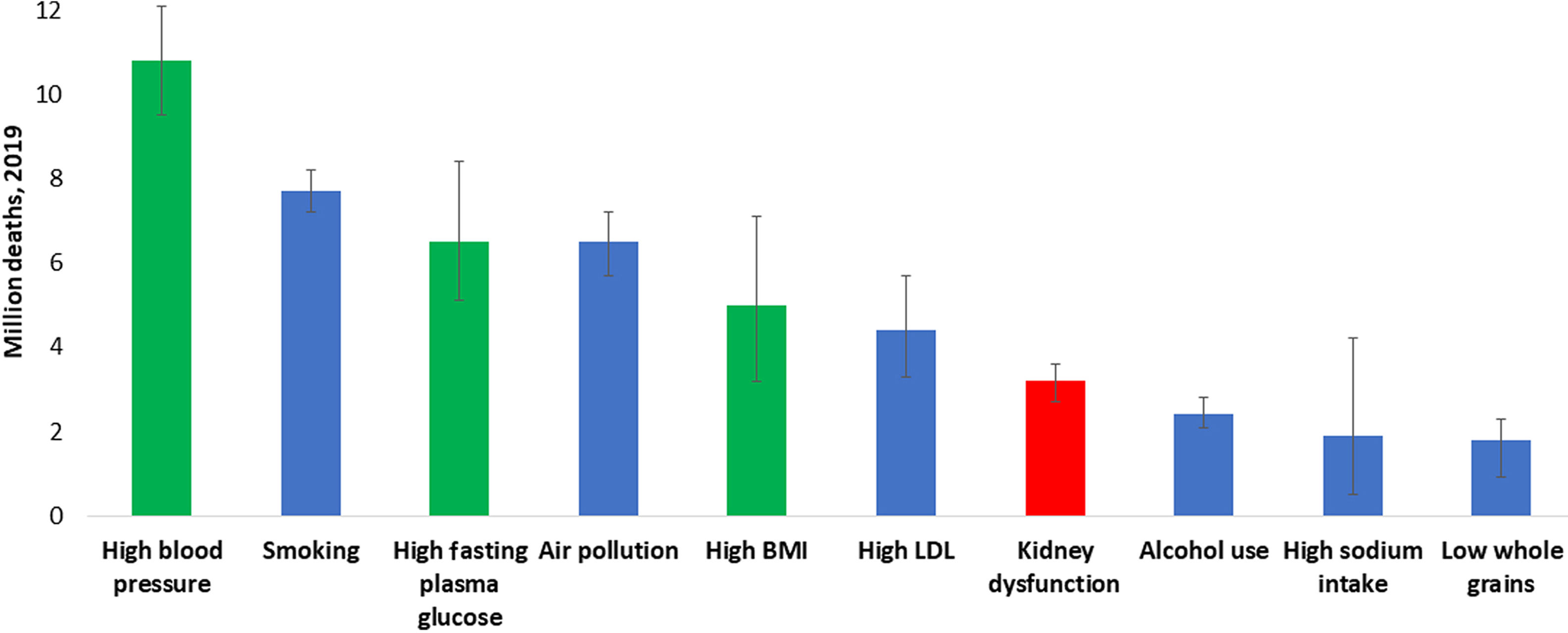
At least 1 in 10 people worldwide is living with kidney disease.1 According to the Global Burden of Disease study, in 2019, >3.1million deaths were attributed to kidney dysfunction, making it the seventh leading risk factor for death worldwide (Fig. 1 and Supplementary Fig. S1).2 However, global mortality from all kidney diseases may actually range between 5 and 11 million per year if the estimated lives lost, especially in lower-resource settings, from acute kidney injury and from lack of access to kidney replacement therapy for kidney failure (KF) are also counted.3 These high global death rates reflect disparities in prevention, early detection, diagnosis, and treatment of chronic kidney disease (CKD).4 Death rates from CKD are especially prominent in some regions, and particularly high in Central Latin America and Oceania (islands of the South Pacific Ocean), indicating the need for urgent action.5
All ages, top 10 global risk factors for death, 2019. Kidney dysfunction (defined as estimated glomerular filtration rate<60ml/min per 1.73m2 or albumin-to-creatinine ratio≥30mg/g) was the seventh leading global level 3 risk factor for death in 2019. The 3 leading global risk factors for kidney disease, including hypertension, diabetes, and overweight/obesity, are also leading global risk factors for death; therefore, holistic strategies are required to address all risk factors simultaneously. Ranking is depicted by millions if deaths are attributed to the risk factors. Error bars depict the confidence range. Global ranking of kidney dysfunction stratified by World Bank income category and gender is shown in Supplementary Fig. S1. Data obtained from the Global Burden of Disease Study.2 BMI, body mass index; LDL, low-density lipoprotein.
CKD also poses a significant global economic burden, with costs increasing exponentially as CKD progresses, not only because of the costs of dialysis and transplantation, but also because of the multiple comorbidities and complications that accumulate over time.6,7 In the United States, Medicare fee-for-service spending for all beneficiaries with CKD was $86.1 billion in 2021 (22.6% of the total expenditure).8 Data from many lower-resource settings are absent, where most costs are paid for out of pocket. A recent study from Vietnam reported that the cost of CKD per patient was higher than the gross domestic product per capita.7 In Australia, it has been estimated that early diagnosis and prevention of CKD could save the health system $10.2 billion over 20 years.9
Although there is regional variation in the causes of CKD, the risk factors with the highest population-attributable factors for age-standardized CKD-related disease-adjusted life years were as follows: high blood pressure (51.4%), high fasting plasma glucose level (30.9%), and high body mass index (26.5%).10 These risk factors are also global leading risk factors for death (Fig. 1). Only 40% and 60% of those with hypertension and diabetes, respectively, are aware of their diagnosis, and far smaller proportions are receiving treatment and at target goals.11,12 Moreover, at least 1 in 5 people with hypertension and 1 in 3 people with diabetes also have CKD.13
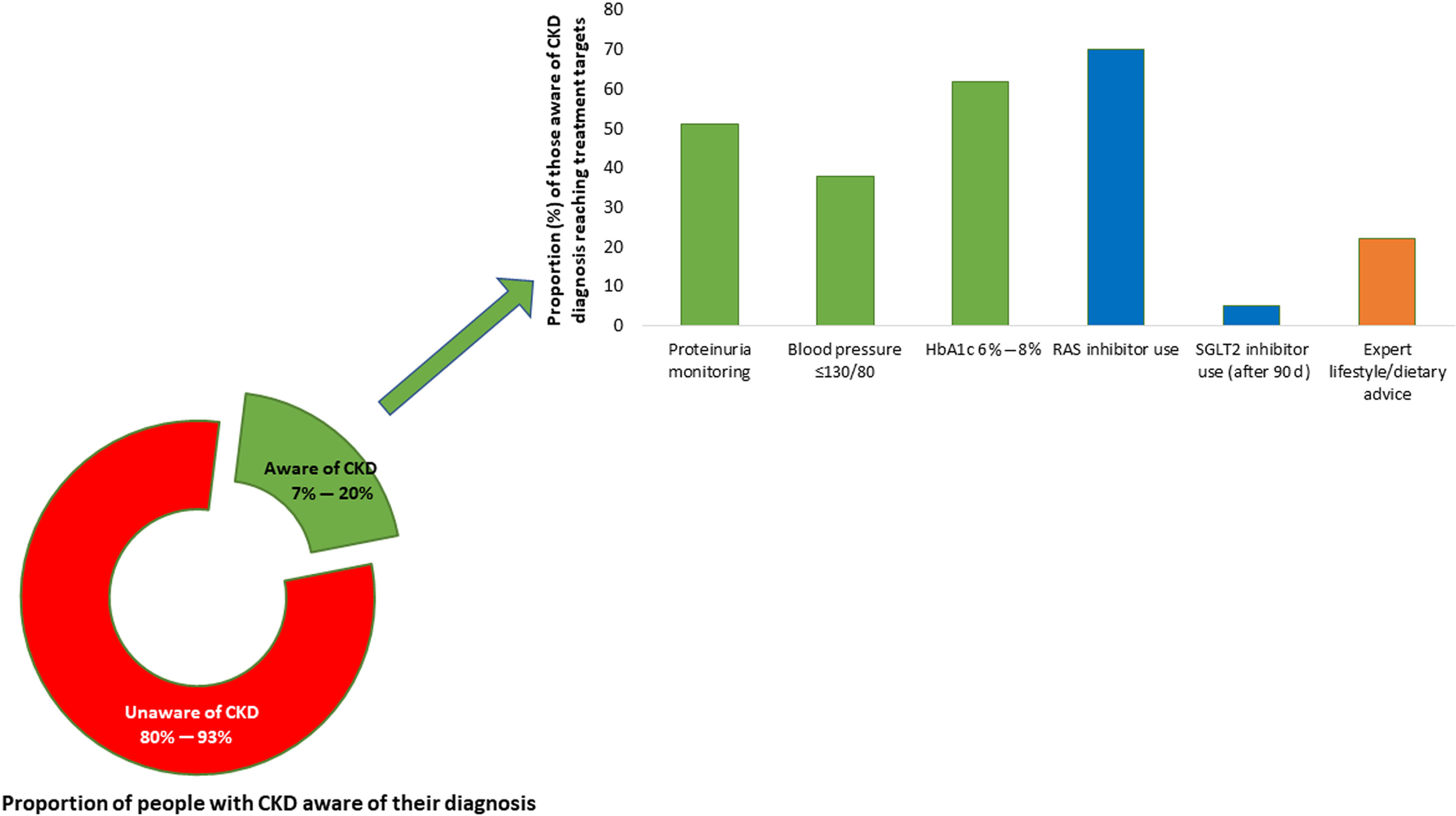
A large proportion of CKD can be prevented through healthy lifestyles, prevention and control of risk factors, avoidance of acute kidney injury, optimization of maternal and child health, mitigation of climate change, and addressing social and structural determinants of health.3 Nevertheless, the benefits of some of these measures may only be seen in generations to come. In the meantime, early diagnosis and risk stratification create opportunities to institute therapies to slow, halt, or even reverse CKD.14 Concerningly, CKD awareness was strikingly low among individuals with kidney dysfunction, with ≈80% to 95% of patients being unaware of their diagnosis across world regions (Fig. 2).15–20 People are dying because of missed opportunities to detect CKD early and deliver optimal care!
Proportion of people with chronic kidney disease (CKD) who are aware of their diagnosis and are receiving appropriate guideline-recommended care. The proportion of people with CKD who are aware of their diagnosis varies globally, with rates ranging from 7% to 20%. As CKD stage worsens, knowledge of CKD increases. Among those with a diagnosis of CKD, the average proportion of patients receiving appropriate medication to delay CKD progression (renin–angiotensin–aldosterone system [RAS] inhibitors and sodium-glucose cotransporter 2 [SGLT2] inhibitors) is suboptimal as are those reaching target blood pressure, diabetes control, and nutrition advice. The treatment targets depicted in the figure follow the Kidney Disease: Improving Global Outcomes (KDIGO) 2012 guidelines.15 Most data come from higher-resource settings; these proportions are likely lower in lower-resource settings. Data are shown for proportions of patients reaching blood pressure of <130/80mm Hg. Data compiled from previous studies.15–20 HbA1c, hemoglobin A1c.
More important, CKD is a major risk factor for cardiovascular disease, and as kidney disease progresses, cardiovascular death and KF become competing risks.21 Indeed, the Global Burden of Disease study data from 2019 showed that more people died of cardiovascular disease attributed to kidney dysfunction (1.7 million people) than from CKD itself (1.4 million people).2 Therefore, cardiovascular disease care must also be a priority for people with CKD.
Gaps between knowledge and implementation in kidney careStrategies to prevent and treat CKD have been built on a strong evidence base over the past 3 decades (Fig. 3).19,22 Clinical practice guidelines for CKD are clear; however, adherence to these guidelines is suboptimal (Fig. 2).15,19,20
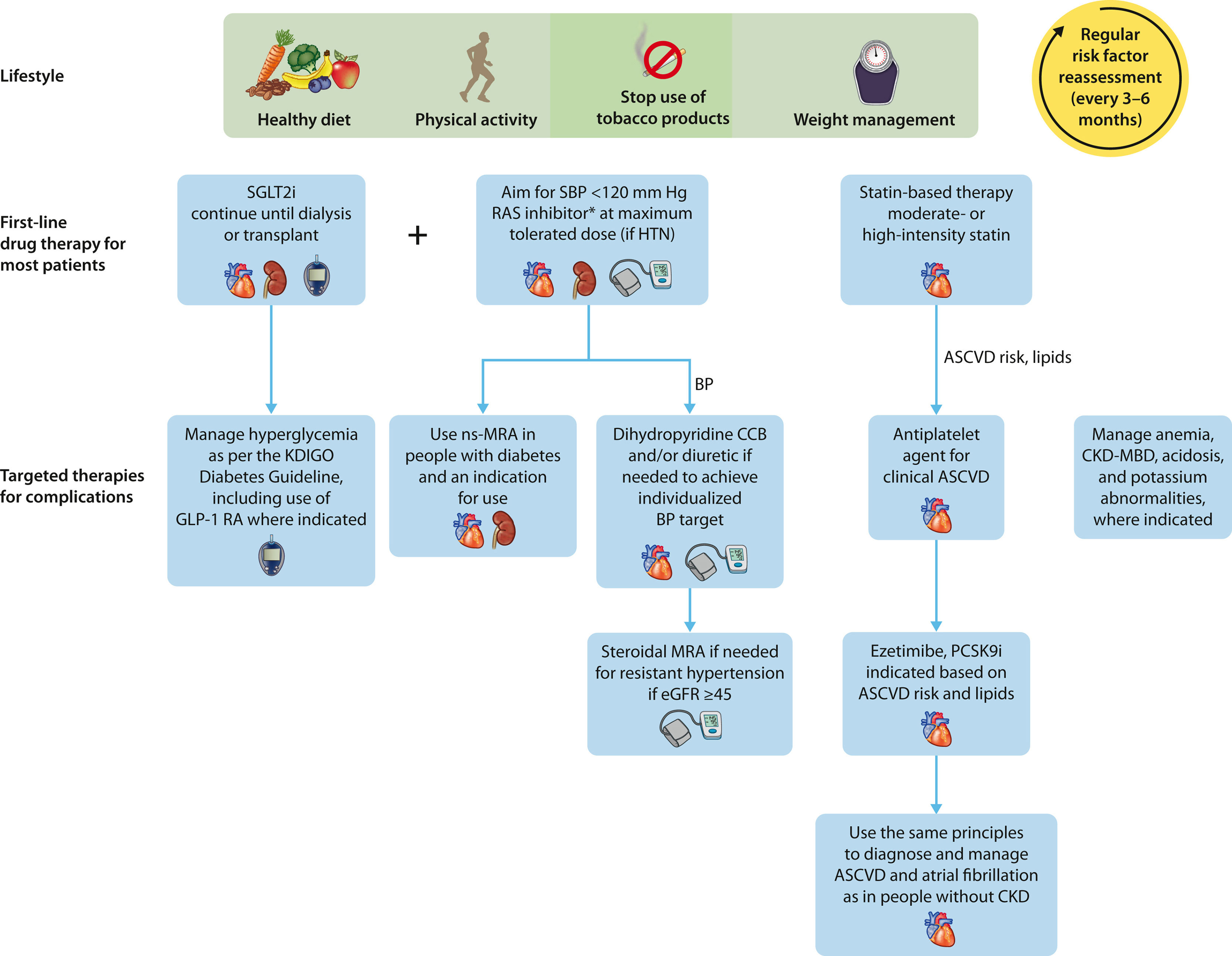
Recommended optimal lifestyle and therapeutic management for chronic kidney disease (CKD) in diabetes. Illustration of a comprehensive and holistic approach to optimizing kidney health in people with CKD. In addition to the cornerstone lifestyle adjustments, attention to diabetes, blood pressure (BP), and cardiovascular risk factor control is intergral to kidney care. *Angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker should be first-line therapy for BP control when albuminuria is present; otherwise dihydropyridine calcium channel blocker (CCB) or diuretic can also be considered. Figure reproduced from Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. https://doi.org/10.1016/j.kint.2023.10.018.22 Copyright © 2023, Kidney Disease: Improving Global Outcomes (KDIGO). Published by Elsevier Inc. on behalf of the International Society of Nephrology under the CC BY-NC-ND license (ht***tp://creativecommons.org/licenses/by-nc-nd/4.0/). ASCVD, atherosclerotic cardiovascular disease; CKD-MBD, chronic kidney disease-mineral and bone disorder; eGFR, estimated glomerular filtration rate; GLP-1 RA, glucagon-like peptide-1 receptor agonist; HTN, hypertension; MRA, mineralocorticoid receptor antagonist; ns-MRA, nonsteroidal mineralocorticoid receptor antagonist; PCSK9i, proprotein convertase subtilisin/kexin type 9 inhibitor; RAS, renin-angiotensin-aldosterone system; SBP, systolic blood pressure; SGLT2i, sodium-glucose cotransporter 2 inhibitor.
Regardless of the cause, control of major risk factors, particularly diabetes and hypertension, forms the foundation of optimal care for CKD.19,23 Beyond lifestyle changes and risk factor control, the initial pharmacologic classes of agents proven to provide kidney protection were the renin–angiotensin–aldosterone system inhibitors in the form of angiotensin-converting enzyme inhibitors (ACEIs) and the angiotensin receptor blockers.14,19 However, despite decades of knowledge that these medications have important protective effects on kidney and heart function in people with CKD, their use has remained low based on real-world data from electronic health records (Fig. 2). For example, in the United States, ACEI or angiotensin receptor blocker use was reported in the range of 20–40% at ≥15 years after the last approvals of these agents for patients with CKD and type 2 diabetes.24 Although more recent data show improvement in prescribing rates to 70% in this population, just 40% persist on an ACEI or angiotensin receptor blocker for at least 90 days.20 These data illustrate gaps in both prescribing kidney protective medication and continuity of care over time, potentially related to cost, lack of patient education, polypharmacy, and adverse effects.25
Although initial enthusiasm for sodium-glucose cotransporter 2 (SGLT2) inhibitors focused on their benefits for diabetes and cardiovascular disease, unprecedented therapeutic benefits have clearly been observed for CKD as well. The relative risk reductions with SGLT2 inhibitors approach 40% for substantial decline in estimated glomerular filtration rate, KF, and death in populations with CKD of several causes, heart failure, or high cardiovascular disease risk.26,27 These benefits accrued on top of standard-of-care risk factor management and renin–angiotensin–aldosterone system inhibitor. Risks of heart failure, cardiovascular death, and all-cause mortality were also reduced in patients with CKD.26 Addition of SGLT2 inhibitor to renin–angiotensin–aldosterone system inhibitors could delay the need for kidney replacement therapy by several years, depending on when they are started.28 Moreover, for every 1000 patients with CKD treated with an SGLT2 inhibitor on top of standard therapy, 83 deaths, 19 heart failure hospitalizations, 51 dialysis initiations, and 39 episodes of acute kidney function worsening can be prevented.29
Concerningly, marked underuse of these and other guideline-recommended therapies, including SGLT2 inhibitors, persists (Fig. 2).20,24 In the CURE-CKD registry, only 5% and 6.3% of eligible patients with CKD and diabetes, respectively, continued on SGLT2 inhibitor and glucagon-like peptide-1 receptor agonist at 90 days.18 Notably, lack of commercial health insurance and treatment in community-based versus academic institutions were associated with lower likelihoods of SGLT2 inhibitor, ACEI, or angiotensin receptor blocker prescriptions among patients with diabetes and CKD.20 In low- or middle-income countries (LMICs), the gap between evidence and implementation is even wider given the high cost and inconsistent availability of these medications, despite availability of generics.30 Such gaps in delivering optimal treatment for CKD are unacceptable.
In addition to the SGLT2 inhibitors, nonsteroidal mineralocorticoid receptor antagonists have been demonstrated to reduce the risks of CKD progression, KF, cardiovascular events, and deaths, on top of the standard of care with renin-angiotensin-aldosterone system inhibitors, in type 2 diabetes.31 A growing portfolio of promising therapeutic options is on the horizon with glucagon-like peptide-1 receptor agonists (NCT03819153, NCT04865770), aldosterone synthase inhibitors (NCT05182840), and dual-to-triple incretins (Supplementary Table S1).26,32 Furthermore, the evidence is already clear that in patients with CKD and diabetes, glucagon-like peptide-1 receptor agonists reduce cardiovascular events, are safe and effective glucose-lowering therapies, and aid with weight loss.32
Historically, it has taken an average of 17 years to move new treatments from clinical evidence to daily practice.33 With millions of people with CKD dying each year, this is far too long to wait.
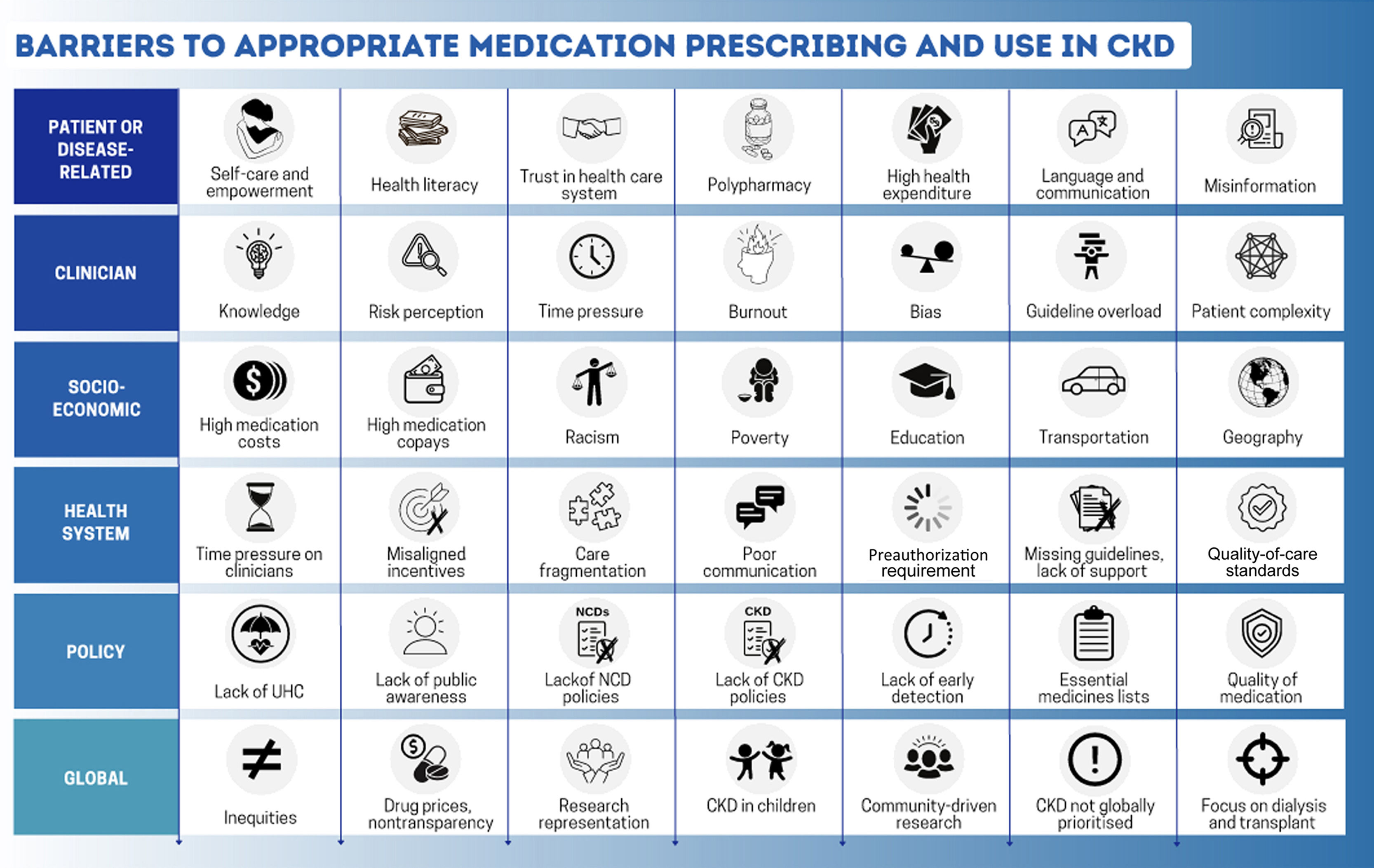
Closing the “gap” between what we know and what we doLack of policies, global inequitiesHealth policySince the launch of the World Health Organization Action Plan for Non-Communicable Diseases (NCDs) in 2013, there has been global progress in the proportion of countries with a national NCD action plan and dedicated NCD units.34 However, CKD is only incorporated into NCD strategies in approximately one-half of countries.4 Policies are required to integrate kidney care within essential health packages under universal health coverage (Fig. 4).30 Multisectoral policies must also address the social determinants of health, which are major amplifiers of CKD risk and severity, limiting people's opportunities to improve their health.3 Lack of investment in kidney health promotion, along with primary and secondary prevention of kidney disease, hinders progress.14
Health systemsTwo major goals of universal health coverage are to achieve coverage of essential health services and reduce financial hardship imposed by health care. However, universal health coverage alone is insufficient to ensure adequate access to kidney care.3 Health systems must be strengthened and quality of care must also be prioritized, as poor quality care contributes to more deaths than lack of access in low-resource settings.35 Quality care requires a well-trained health care workforce, sustainable availability of accurate diagnostics, reliable infrastructure, and medication supplies and should be monitored in an ongoing process of quality improvement (Fig. 4). The quality of medications, especially in LMICs, may be an additional barrier to successful management of CKD.36 Regulation and monitoring of drug manufacturing and quality standards are important to ensure safe and effective therapies. Strategies to support regulation and quality assurance will need to be developed in local contexts and guidance, as outlined elsewhere.37
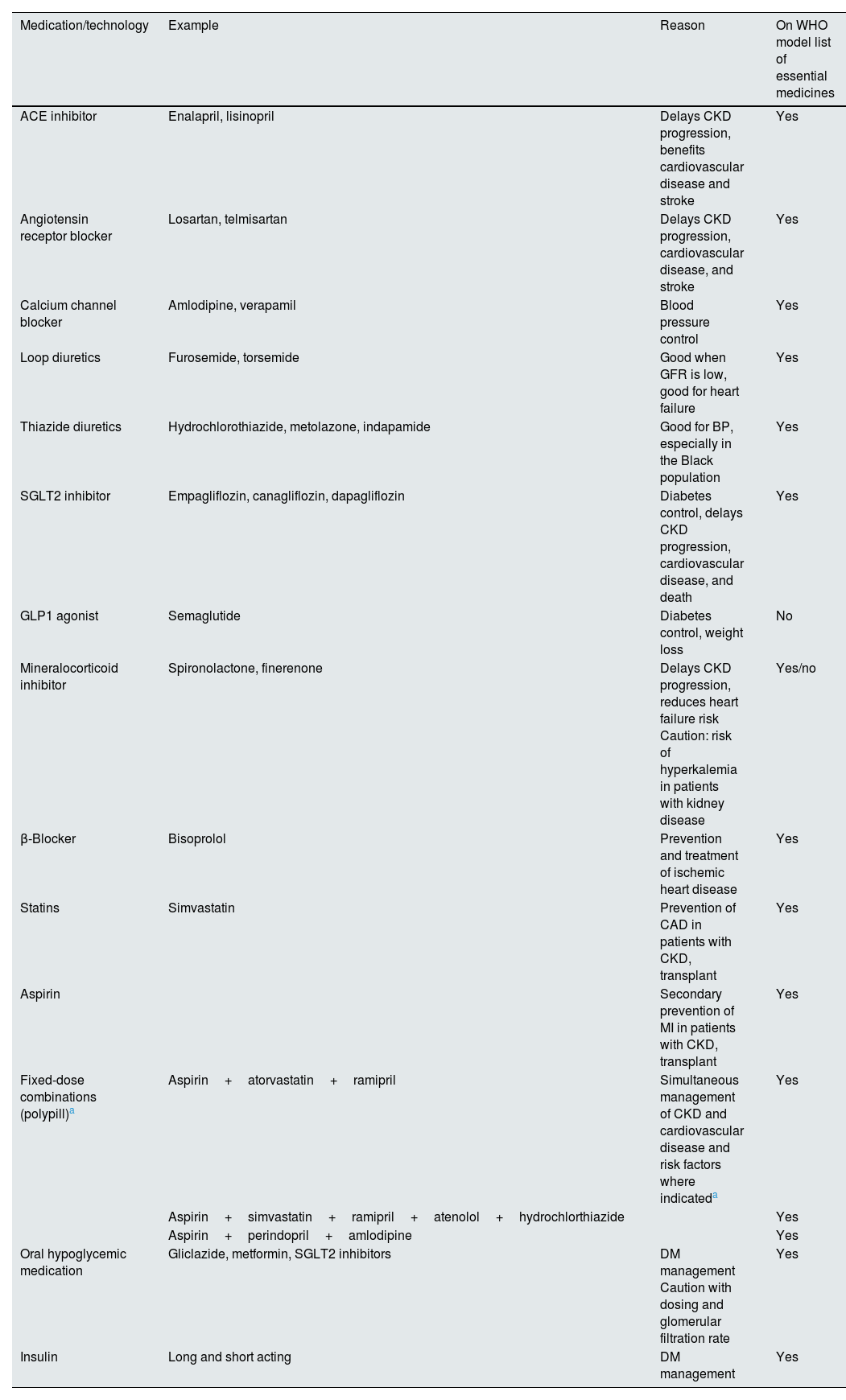
Establishing a credible case for CKD detection and management based on risks, interventions and outcomes, and costs, based on real-world data, will help to translate theoretical cost-effectiveness (currently established primarily in high-income countries with minimal data from elsewhere) into economic reality.30,38 Screening should include evaluation of risk factors for CKD, eliciting a family history, recognizing potential symptoms (usually advanced—fatigue, poor appetite, edema, itching etc.), and measuring blood pressure, serum creatinine, urinalysis, and urine albumin/protein to creatinine ratios, as outlined in established guidelines.19,39 Addressing CKD upstream beginning in primary care should lower costs over time by reducing CKD complications and KF. Medications required for kidney care are already included in the World Health Organization Essential Medication List (Table 1). These must be provided at national levels under universal health coverage.40 Pharmaceutical companies should provide these at affordable prices.
Essential medicines for patients with kidney disease.
| Medication/technology | Example | Reason | On WHO model list of essential medicines |
|---|---|---|---|
| ACE inhibitor | Enalapril, lisinopril | Delays CKD progression, benefits cardiovascular disease and stroke | Yes |
| Angiotensin receptor blocker | Losartan, telmisartan | Delays CKD progression, cardiovascular disease, and stroke | Yes |
| Calcium channel blocker | Amlodipine, verapamil | Blood pressure control | Yes |
| Loop diuretics | Furosemide, torsemide | Good when GFR is low, good for heart failure | Yes |
| Thiazide diuretics | Hydrochlorothiazide, metolazone, indapamide | Good for BP, especially in the Black population | Yes |
| SGLT2 inhibitor | Empagliflozin, canagliflozin, dapagliflozin | Diabetes control, delays CKD progression, cardiovascular disease, and death | Yes |
| GLP1 agonist | Semaglutide | Diabetes control, weight loss | No |
| Mineralocorticoid inhibitor | Spironolactone, finerenone | Delays CKD progression, reduces heart failure risk Caution: risk of hyperkalemia in patients with kidney disease | Yes/no |
| β-Blocker | Bisoprolol | Prevention and treatment of ischemic heart disease | Yes |
| Statins | Simvastatin | Prevention of CAD in patients with CKD, transplant | Yes |
| Aspirin | Secondary prevention of MI in patients with CKD, transplant | Yes | |
| Fixed-dose combinations (polypill)a | Aspirin+atorvastatin+ramipril | Simultaneous management of CKD and cardiovascular disease and risk factors where indicateda | Yes |
| Aspirin+simvastatin+ramipril+atenolol+hydrochlorthiazide | Yes | ||
| Aspirin+perindopril+amlodipine | Yes | ||
| Oral hypoglycemic medication | Gliclazide, metformin, SGLT2 inhibitors | DM management Caution with dosing and glomerular filtration rate | Yes |
| Insulin | Long and short acting | DM management | Yes |
ACE, angiotensin-converting enzyme; BP, blood pressure; CAD, coronary artery disease; CKD, chronic kidney disease; DM, diabetes mellitus; GFR, glomerular filtration rate; GLP1, glucagon-like peptide-1; MI, myocardial infarction; SGLT2, sodium-glucose cotransporter 2; WHO, World Health Organization.
A shortage of primary care professionals is compounded by inconsistent access to specialists and allied health professionals in both high-income countries and LMICs. Defining roles and responsibilities for kidney care is essential. Solutions may include multidisciplinary team care (primary care physicians, pharmacists, advanced practitioners, nurses, therapists, educators, nutritionists, and mental health professionals) with well-established mechanisms of collaboration of all elements and promptly available communication technologies within health systems and between professionals to support care and decision-making.41,42 Brain drain in low-resource settings is complex and must be tackled.
Mobilization of community health workers yields cost savings in infectious disease programs in LMICs, and may facilitate early detection, diagnosis, and management of NCDs.43 Protocolized CKD management, possibly supported by electronic decision-support systems, lends itself well to interventions at the community level, with integration of primary care physicians and backup from nephrology and other professionals.44,45 In some environments, pharmacists, for example, could identify people with diabetes or hypertension, at risk of CKD, based on their prescriptions, and could offer testing on site and reference if needed.46 Pharmacists can also provide medication reconciliation and medication advice for safety, effectiveness, and adherence. Social workers and pharmacists can help patients with medications access programs.46
Challenges for clinical inertiaClinical “inertia,” commonly blamed for low prescribing rates, has many facets (Fig. 4).47 Many knowledge gaps regarding CKD exist among primary care clinicians.48 Such gaps are remediable with focused public and professional education. Additional factors include fear of medication adverse effects, misaligned incentives within the health system, excessive workload, formulary restrictions, and clinician burnout.47 Furthermore, discrepancies in guideline recommendations from different professional organizations may add to confusion. A major impediment to optimal care is the time constraints imposed on individual clinicians. The average primary care practitioner in the United States would require ≈26.7h per day to implement guideline-recommended care for a 2500 patient panel.49 Innovation is required to support guideline implementation, especially for primary care practitioners who must implement many different guidelines to meet the needs of various patients. Electronic health records, reminders, team-based nudges, and decision support tools offer a promising support for quality kidney care in busy clinical practices.50 The extra time and effort spent negotiating preauthorizations or completing medication assistance program requests, along with need for frequent monitoring of multiple medications, however, also hinder appropriate prescribing.25 Many primary care practitioners have only a few minutes allocated per patient because of institutional pressure or patient volume. “Inertia” can hardly be applied to clinicians working at this pace. The number of health professionals must increase globally.
Visits for patients with CKD are complex as multimorbidity is high. Patients are often managed by multiple specialists, leading to fragmentation of care, lack of holistic oversight, and diffusion of responsibility for treatment. Multidisciplinary care improved transition to kidney replacement therapy and lowered mortality in single and combined outcomes analyses.51 Novel models of “combined clinics” with on-site collaboration and coparticipation (nephrologist–cardiologist–endocrinologist) may prove to be of substantial benefit for patients, in terms of reduced fragmentation of care, logistics, and cost saving.
Patient centerednessHealth literacySelf-care is the most important aspect of kidney care. A patient's ability to understand his/her health needs, make healthy choices, and feel safe and respected in the health system, and psychosocial support are important to promote health decision-making (Fig. 4). Communication should start from good communication that requires quality information and importantly confirmation of “understanding” on the side of the patient and often family. Electronic apps and reminders may become useful tools to support patients by improving disease knowledge, promoting patient empowerment, and improving self-efficacy, although it is unlikely that one size will fit all.52 Insufficient patient health information, poor communication, and mistrust, among other elements, are important barriers, especially in marginalized and minoritized communities, where CKD is common.30 Patients may also be confused by contradictory recommendations for care between health care professionals, as well as conflicting messaging in lay media. Innovative platforms to improve communication between patients and clinicians about CKD are promising and may promote optimal prescribing and adherence.53,54
To overcome barriers and promote equity, patient perspectives are essential to designing and testing better health strategies. Collaborative care models must include patients, families, community groups, diverse health care professionals, health systems, government agencies, and payers.38 Advocacy organizations and local community groups and peer navigators, having trusted voices and relationships, can be conduits for education and may provide input for development of patient tools and outreach programs.55 Most important, patients must be at the center of their care.
Cost and availability of medicationIn high-income countries, people without health insurance and those with high copays paradoxically pay the most for even essential medications.38 Across LMICs, kidney disease is the leading cause of catastrophic health expenditure because of reliance on out-of-pocket payments.56 Across 18 countries, 4 cardiovascular disease medications (statins, ACEIs, aspirin, and β-blockers), all often indicated in CKD, were more available in private than in public settings, mostly unavailable in rural communities, and unaffordable for 25% of people in upper middle-income countries and 60% of people in low-income countries.57 Newer therapies may be prohibitively expensive worldwide, especially where generics may not yet be available. In the United States, the retail price for a 1-month supply of an SGLT2 inhibitor or finerenone is ≈$500–$700; and for glucagon-like peptide-1 receptor agonists, ≈$800–$1200 per month.38 Reliance on out-of-pocket payment for vital, life-saving basic medications is unacceptable (Fig. 4).
Special considerationsNot all kidney diseases are the same. Much of what has been discussed here relates to the most common forms of CKD (e.g. diabetes and hypertension). Some forms of CKD not yet completely understood have different risk profiles, including environmental exposures, genetic predisposition, and autoimmune or other systemic disorders. Highly specialized therapies may be required. Pharmaceutical companies should be accountable to ensure that research studies include disease-representative participants with appropriate race, ethnicity, and sex and gender representation, that effective drugs are made available after studies, and that the balance between profit and prices is fair and transparent. Many novel therapies are offering new hope for diverse kidney diseases; and once approved, there must be no delay in extending the benefits to all affected patients (Supplementary Table S1).
An important group often overlooked is children with kidney diseases. This group is especially vulnerable in LMICs, where nephrology services and resources are limited, and families must often make the choice to pay for treatment for 1 child or support the rest of their family.58 Children with CKD are also at high risk of cardiovascular disease, even in high-income settings, and more attention is required to control risk factors and achieve treatment targets.59
Fostering innovationImplementation science and knowledge translationGiven that we know how to treat CKD based on a rigorous evidence base, we must now optimize implementation.60 Implementation research aims to identify effective solutions by understanding how evidence-based practices, often developed in high-income countries, can be integrated into care pathways in lower-resource settings. The management of CKD lends itself to implementation research: optimal therapeutic strategies are known, outcomes are easily measurable, and essential diagnostics and medications should already be in place. Eliciting local patient preferences and understanding challenges are crucial components of such research. Ministries of health should commit to overcoming identified barriers and scaling up successful and sustainable programs.
Polypills as an example of simple innovationPolypills are attractive on multiple levels: fixed doses of several guideline-recommended medications are present within 1 tablet (Table 1); lower price; reduced pill burden; and simplicity of the regimen.61 Polypills have been shown to prevent cardiovascular disease, and to be cost-effective for patients with CKD.62 More studies are needed, but given the alternatives of costly kidney replacement therapy or early death, it is likely that polypills will prove cost-effective to reduce CKD progression.
Harnessing digital technologiesIntegration of telehealth and other types of remotely delivered care can improve efficiency and reduce costs.63 Electronic health records and registries can support monitoring of quality of care and identify gaps to guide implementation and improve outcomes within learning health care systems. Artificial intelligence may also be harnessed to risk stratify and personalize medication prescribing and adherence.64 The use of telenephrology for communication between primary care and subspecialists may also prove of use and benefit for patient treatment.65
Patient perspectivesMultiple methods support elicitation of patient preferences for CKD care, including interviews, focus groups, surveys, discrete choice experiments, structured tools, and simple conversations.66,67 At present, many of these are in research stages. Translation into the clinic will require contextualization and determination of local and individual acceptability.
The journey of each person living with CKD is unique; however, challenges and barriers exist in common. As examples of lived experiences, comments solicited from patients about their medications and care are outlined in Box 1 and Supplementary Table S2. These voices must be heard and headed to close gaps and improve quality of kidney care everywhere.
Barriers impacting medication use as expressed by people living with kidney disease.
“I have to pay for my medications so I either settle for less expensive options or ration the regular dose.”
“I am seeing doctors of different specialties each of whom prescribe separate regimens which makes me concerned about drug interactions.”
“As an experienced patient, I sometimes stop, or modify the dose of the prescribed medications without referring to my doctors. If they do ask, I would tell them that I am in full compliance.”
“Over time, the dose and varieties of my medication keep increasing. I am not sure whether it's because of condition worsening or medications becoming less effectiveness.”
“My knowledge of medication mostly comes from a peer patient who appears to be very knowledgeable about this stuff.”
A stalemate in kidney care has been tolerated far too long. The new therapeutic advances offer real hope that many people with CKD can survive without developing KF. The evidence of clinical benefit is overwhelming and unequivocal. We cannot wait another 17 years for this evidence to trickle into clinical practice.33 The time is now to ensure that all who are eligible to receive CKD treatment equitably receive this care.
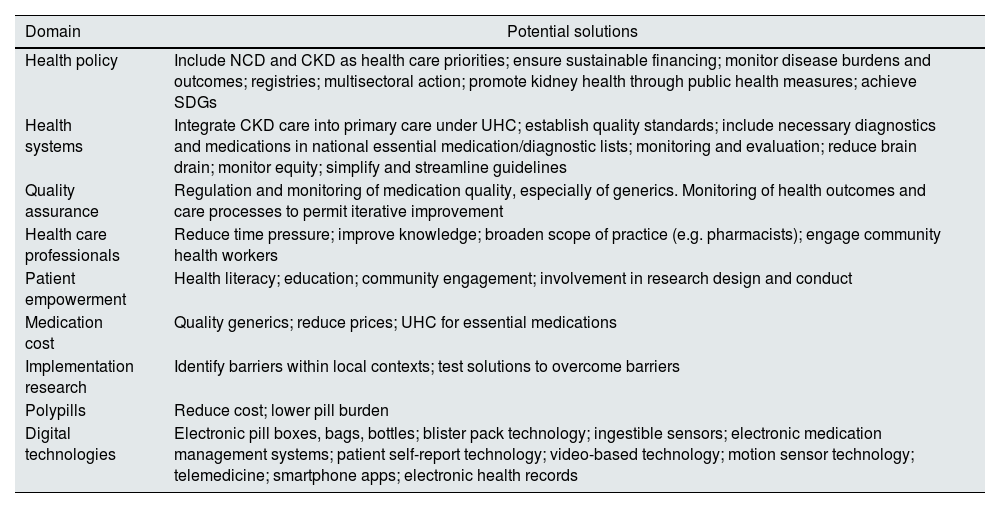
Known barriers and global disparities in access to diagnosis and treatment must be urgently addressed (Fig. 4). To achieve health equity for people with and at risk of kidney diseases, we must raise awareness from policy makers to patients and the general population, harness innovative strategies to support all cadres of health care workers, and balance profits with reasonable prices (Table 2). If we narrow the gap between what we know and what we do, kidney health will become a reality worldwide.
Examples of strategies to improve implementation of appropriate CKD care.
| Domain | Potential solutions |
|---|---|
| Health policy | Include NCD and CKD as health care priorities; ensure sustainable financing; monitor disease burdens and outcomes; registries; multisectoral action; promote kidney health through public health measures; achieve SDGs |
| Health systems | Integrate CKD care into primary care under UHC; establish quality standards; include necessary diagnostics and medications in national essential medication/diagnostic lists; monitoring and evaluation; reduce brain drain; monitor equity; simplify and streamline guidelines |
| Quality assurance | Regulation and monitoring of medication quality, especially of generics. Monitoring of health outcomes and care processes to permit iterative improvement |
| Health care professionals | Reduce time pressure; improve knowledge; broaden scope of practice (e.g. pharmacists); engage community health workers |
| Patient empowerment | Health literacy; education; community engagement; involvement in research design and conduct |
| Medication cost | Quality generics; reduce prices; UHC for essential medications |
| Implementation research | Identify barriers within local contexts; test solutions to overcome barriers |
| Polypills | Reduce cost; lower pill burden |
| Digital technologies | Electronic pill boxes, bags, bottles; blister pack technology; ingestible sensors; electronic medication management systems; patient self-report technology; video-based technology; motion sensor technology; telemedicine; smartphone apps; electronic health records |
CKD, chronic kidney disease; NCD, noncommunicable disease; SDG, sustainable development goal; UHC, universal health coverage.
V.A.L. and K.R.T. first draft/review of draft, and all other authors – D.A., R.C.-R., W.W.S.F., A.H., L.-L.H., M.K., L.A.K., F.L., V.R., S.R., M.S., I.U., B.W., S.-F.L., V.L., A.B. resources/review of draft.
Ethics approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Availability of data and materialAll data are available within the paper.
FundingNone.
Conflict of interestsNone.
The authors are grateful for the thoughtful input provided by members of the Advocacy Working Group and the Patient Liaison Advisory Group of the International Society of Nephrology: Elliot Tannor, Marcello Tonelli, Boris Bikbov, Maria Carlota Gonzalez, Vivekanand Jha, and Viviane Calice-Silva.
VL is chair of the Advocacy Working Group, International Society of Nephrology, no financial disclosures. KRT has received research grants from the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases, National Heart, Lung, and Blood Institute, National Center for Advancing Translational Sciences, National Institute on Minority Health and Health Disparities, director's office), the US Centers for Disease Control and Prevention, and Travere Therapeutics; and consultancy fees from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk. She is chair of the Diabetic Kidney Disease Collaborative for the American Society of Nephrology. RC-R is a member of the Steering Committee of World Kidney Day, a member of the Diabetes Committee of the Latin-American Society of Nephrology and Hypertension (SLANH), and a member of the Latin American Regional Board, International Society of Nephrology. He is a member of the Steering Committee of the Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trial (AstraZeneca), the Study of Diabetic Nephropathy with Atrasentan (SONAR) (Abbvie), A Non-interventional Study Providing Insights Into the Use of Finerenone in a Routine Clinical Setting (FINE-REAL) (Bayer), and CKD-ASI (Boehringer). He has received research grants from AstraZeneca, GlaxoSmithKline, Roche, Boehringer, and Novo Nordisk; and has received honoraria as a speaker from AstraZeneca, Bayer, Boehringer Ingelheim, and Amgen. All the other authors declared no competing interests.
This article is being published in Kidney International and is being reprinted in several journals. The articles cover identical concepts and wording, but vary in minor stylistic and spelling changes, detail, and length of manuscript in keeping with each journal's style. Any of these versions may be used in citing this article.
VAL and KRT are joint first authors.
- Home
- All contents
- Publish your article
- About the journal
- Metrics
- Open access
- Download PDF
- Bibliography
- Additional material



![Proportion of people with chronic kidney disease (CKD) who are aware of their diagnosis and are receiving appropriate guideline-recommended care. The proportion of people with CKD who are aware of their diagnosis varies globally, with rates ranging from 7% to 20%. As CKD stage worsens, knowledge of CKD increases. Among those with a diagnosis of CKD, the average proportion of patients receiving appropriate medication to delay CKD progression (renin–angiotensin–aldosterone system [RAS] inhibitors and sodium-glucose cotransporter 2 [SGLT2] inhibitors) is suboptimal as are those reaching target blood pressure, diabetes control, and nutrition advice. The treatment targets depicted in the figure follow the Kidney Disease: Improving Global Outcomes (KDIGO) 2012 guidelines.15 Most data come from higher-resource settings; these proportions are likely lower in lower-resource settings. Data are shown for proportions of patients reaching blood pressure of <130/80mm Hg. Data compiled from previous studies.15–20 HbA1c, hemoglobin A1c. Proportion of people with chronic kidney disease (CKD) who are aware of their diagnosis and are receiving appropriate guideline-recommended care. The proportion of people with CKD who are aware of their diagnosis varies globally, with rates ranging from 7% to 20%. As CKD stage worsens, knowledge of CKD increases. Among those with a diagnosis of CKD, the average proportion of patients receiving appropriate medication to delay CKD progression (renin–angiotensin–aldosterone system [RAS] inhibitors and sodium-glucose cotransporter 2 [SGLT2] inhibitors) is suboptimal as are those reaching target blood pressure, diabetes control, and nutrition advice. The treatment targets depicted in the figure follow the Kidney Disease: Improving Global Outcomes (KDIGO) 2012 guidelines.15 Most data come from higher-resource settings; these proportions are likely lower in lower-resource settings. Data are shown for proportions of patients reaching blood pressure of <130/80mm Hg. Data compiled from previous studies.15–20 HbA1c, hemoglobin A1c.](https://static.elsevier.es/multimedia/20132514/0000004400000005/v1_202411140702/S2013251424001974/v1_202411140702/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w94GCRvdQBB6xyQjMrWMzrts=)






