La proteinuria es el principal predictor de progresión de la enfermedad renal crónica. Los fármacos que bloquean el eje renina-angiotensina-aldosterona (RAA) reducen la proteinuria y retrasan la progresión de la enfermedad. Sin embargo, su efecto es subóptimo, y la proteinuria residual persiste como predictor relevante de deterioro renal. La vitamina D tiene efectos pleiotrópicos que podrían impactar en estos parámetros. En este trabajo revisamos críticamente las bases moleculares y experimentales que sugieren un efecto antiproteinúrico de la activación del receptor de vitamina D (VDR), así como la evidencia disponible sobre su efecto antiproteinúrico en la práctica clínica. En modelos animales se ha observado un efecto antiproteinúrico de la activación del VDR, que podría deberse a una acción protectora directa sobre el podocito u otros efectos pleiotrópicos que frenen la activación del sistema RAA, la inflamación y la fibrosis. Los ensayos clínicos se han realizado en general en pacientes con déficit o insuficiencia de vitamina D y el mayor de ellos (VITAL) no demostró que el paricalcitol mejorara el objetivo primario del estudio (descenso del cociente albúmina creatinina urinario). En este sentido, la información disponible es insuficiente para aconsejar el empleo de la vitamina D nativa o de activadores del VDR como fármacos antiproteinúricos renoprotectores más allá del ámbito experimental. Dos ensayos clínicos españoles y uno italiano intentan aclarar cuál es el efecto del paricalcitol y la vitamina D sobre la proteinuria residual en diversas circunstancias clínicas (PALIFE, NEFROVID y PROCEED).
Proteinuria is the main predictor of chronic kidney disease progression. Drugs that block the renin-angiotensin-aldosterone system (ARBs) reduce proteinuria and slow down the progression of the disease. However, their effect is suboptimal, and residual proteinuria persists as an important predictor of renal impairment. Vitamin D has pleiotropic effects that could have an impact on these parameters. In this study, we critically review the molecular and experimental bases that suggest an antiproteinuric effect of vitamin D receptor (VDR) activation and the available evidence on its antiproteinuric effect in clinical practice. In animal models, we have observed the antiproteinuric effect of VDR activation, which could be due to direct protective action on the podocyte or other pleiotropic effects that slow down RAA system activation, inflammation and fibrosis. Clinical trials have generally been conducted in patients with a vitamin D deficiency or insufficiency and the main trial (VITAL) did not demonstrate that paricalcitol improved the study’s primary endpoint (decrease in the urine albumin to creatinine ratio). In this sense, the information available is insufficient to advise the use of native vitamin D or VDR activators as renoprotective antiproteinuric drugs beyond the experimental level. Two Spanish clinical trials and one Italian trial attempted to determine the effect of paricalcitol and vitamin D on residual proteinuria in various clinical circumstances (PALIFE, NEFROVID and PROCEED).
Contents
Proteinuria is the main predictor of chronic kidney disease (CKD) progression and, although it is a surrogate biochemical parameter, it is now recognised as the first therapeutic target in the management of CKD.1-5
Drugs that block the renin-angiotensin-aldosterone system (ARBs) have proven to be effective in reducing proteinuria and slowing down progression of the disease. After implementing the initial hygiene and dietary recommendations, ARBs are the first step in renoprotective antiproteinuric treatment and are graded as level 1 evidence.3,4,6-11 However, their antiproteinuric effect is usually suboptimal, and residual proteinuria continues to be a target for treatment, with additional renoprotective effects being provided.10,11 Although ARBs may reduce residual proteinuria, dual blockade has potential adverse effects, it does not decrease mortality and it has not been shown to improve the development of kidney function in prevalent nephropathies such as diabetes.12-14 Therefore, neither the recent recommendations of the American Diabetes Association 2013 on diabetic nephropathy15 or the KDIGO (Kidney Disease: Improving Global Outcomes)1 guidelines recommend its use.
Clinically, many drugs have been tested to reduce residual proteinuria and others are being tested. In this regard, vitamin D and its derivatives have shown a broad range of pleiotropic or multisystemic effects on the kidneys, the cardiovascular system and the immune response. The antiproteinuric effect is one of them and there are numerous experimental data to support this, but it has not been clinically demonstrated.16-19
The aim of this review is to analyse the biological bases and degree of evidence in the clinical setting for the use of vitamin D and vitamin D receptor (VDR) agonists as potential antiproteinuric drugs.
MOLECULAR BASES AND LESSONS FORM BASIC EXPERIMENTATION
Pathogenesis of proteinuria
The glomerular filtration barrier consists of three layers: the fenestrated capillary endothelium, the glomerular basement membrane and the podocytes in the outermost layer. The podocyte plays the main role in maintaining the glomerular filtration barrier and glomerular structural integrity. The podocytes are terminally differentiated cells that have exhausted their proliferative capacity. Therefore, lesions to or loss of podocytes can result in irreversible glomerular lesions, with progression to focal segmental glomerulosclerosis, manifested clinically as proteinuria and possible loss of renal function.20
In normal conditions, plasma is filtered into Bowman’s space between the foot processes of the podocytes, crossing the slit diaphragm, which retains proteins. The central component of the slit diaphragm is the nephrin, a protein identified in 1998 by cloning the mutated gene in congenital nephrotic syndrome of the Finnish type, a condition characterised by massive proteinuria.21 Nephrin has a structural and signalling role, and together with the other components of the slit diaphragm, they form a porous molecular sieve that is the main element responsible for the retention of proteins.22,23
Furthermore, the podocyte expresses numerous receptors and responds to the presence of growth factors and metabolic products involved in CKD. Among other receptors, it has angiotensin 2 receptors, transforming growth factor beta 1 (TGFβ1) and VDR.20,24
Vitamin D
Natural or native vitamin D is a product of diet or synthesis in the skin. Diet provides two forms of vitamin D: ergocalciferol (D2) and cholecalciferol (D3). In skin, D3 is synthesised in response to stimulation by the sun’s ultraviolet rays. The D2 and D3 forms are modified to become the active form of vitamin D. In the liver, the enzyme 25-hydroxylase converts them into calcidiol or 25-hydroxy-vitamin D (25OHD). The enzyme renal and extrarenal 1 alpha hydroxylase transforms calcidiol into calcitriol or 1,25-dihydroxyvitamin D (1,25 OHD). Calcitriol is an activator of the intracellular receptor VDR (VDRA). In addition to these natural products, there are selective VDRA drugs (sVDRA), such as paricalcitol.
In practical terms, the actions of vitamin D can be subdivided into calciotropic and non-calciotropic.25 Calciotropic actions are related to VDR activation by endocrine calcitriol from the proximal tubule. These actions regulate mineral metabolism by acting on the parathyroid glands, bone and the intestines to modulate the secretion of PTH, bone resorption and intestinal absorption of calcium and phosphorus.
Non-calciotropic or pleiotropic actions are related to autocrine or paracrine activation of VDR by calcitriol of extrarenal origin, acting on the immune system, pancreas, heart and other organs. There are also autocrine and paracrine actions of calcitriol in the kidney itself, not related to mineral metabolism. Therefore, in addition to the large endocrine calcitriol production in the proximal tubule, podocytes are also capable of synthesising calcitriol for their own use and they express both 1-alpha-hydroxylase and VDR.26
Vitamin D action requires VDR activation. The VDR-ligand complex behaves as a transcription factor which can promote or suppress gene transcription, favouring or preventing the production of messenger RNA and the subsequent translation and protein formation.27 The specific pattern of genes activated or suppressed depends on the vitamin D dose, the target cell, the cell microenvironment and the specific ligand.
The relative importance of these factors has been studied in cultured smooth muscle cells using a transcriptomic approach, providing an overall view of the cell response. Scientists compared the effect of calcitriol with that of paricalcitol and also studied the effect of extracellular phosphate concentration.28,29 In this experimental system and at the concentrations (0.1 microM) and times studied (30 hours), no significant differences were observed between overall gene expression in response to calcitriol or paricalcitol. Both drugs inhibited cell proliferation in a manner that was dependent on the dose.28 By contrast, the environment, represented by extracellular phosphate concentration, had a greater impact. Increasing the phosphate concentration exerted a generalised effect on VDR-mediated gene expression, affecting the number of genes modulated.29
It is possible that the effect of phosphorus concentration may be more important in vivo than the specific VDR ligand being used. Thus, paricalcitol appears to be associated with a lower risk of hypercalcaemia and hyperphosphataemia than calcitriol.30 In this sense, there is growing evidence on the role of the extracellular phosphorus concentration on the podocyte and proteinuria. In 331 patients with proteinuric nephropathies treated with the angiotensin converting enzyme inhibitor ramipril, CKD progressed faster in patients with higher baseline serum phosphate levels, which suggests that high phosphate levels decrease the renoprotective effect of ramipril.31 Likewise, the overexpression of a phosphate transporter in rats induces phosphate-dependent podocyte lesion, which contributes to the progression of glomerular sclerosis in the kidney.32
Therapeutic use of VDR agonists in the treatment of proteinuria requires a precise knowledge of the dose required to obtain antiproteinuric effects avoiding hypercalcaemia and vascular calcification. The strategy of administering VDR agonists systemically, imitating the regulating endocrine action of calcium-phosphorus metabolism to achieve autocrine or paracrine (pleiotropic) effects has the potential danger of triggering adverse effects related to the system’s calciotropic actions.
In this sense, the existence of calciotropic actions depends on the dose used both for calcitriol and paricalcitol. The effect of the dose has been well defined in animals in experiments, in which the higher doses of both compounds cause hypercalcaemia, hyperphosphataemia and vascular calcification.30 It is necessary to find a balance between the benefits and adverse effects of vitamin D, optimising the dose to obtain the maximum benefits with minimal adverse effects. Treatment with vitamin D is accepted in patients with renal disease, mainly because of the widely recognised benefits associated with the regulation of calcium-phosphorus metabolism, to which the potential pleiotropic benefits mentioned above are added. However, there is still not enough long-term experience to define the risk of vascular calcification with higher doses of the newer drugs.
In a study which evaluated the effect of treatment with calcitriol and paricalcitol on vascular smooth muscle cells both in vivo and in vitro in rats with advanced CKD, cells cultured with calcitriol developed calcification but this effect was not observed in cells cultured with paricalcitol. We also observed increased pulse pressure only in animals treated with calcitriol. This may be explained by the increase in vascular calcification, and this effect was independent of the levels of serum calcium and phosphorus, as similar levels were obtained with both drugs.30 However, paricalcitol-treated animals also developed hypercalcaemia and a fivefold increase in the magnitude of vascular calcification compared to controls without vitamin D. In another study in rats with CKD, both low-dose calcitriol and paricalcitol protected against aortic calcification, but in high doses both drugs stimulated vascular calcification.33
Vitamin D and podocytes
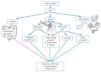
VDR activation could have antiproteinuric and renoprotective effects, acting at various levels, including direct effects on podocytes (Figure 1).
Podocytes express both 1-α-hydroxylase and VDR in culture and in vivo,26 giving them the ability both to produce calcitriol and respond to autocrine or endocrine calcitriol.
In cultured podocytes, calcitriol activated the transcription of the nephrin gene in a manner that was dependent on the dose.34 This could favour preservation of retention of proteins. The experimental system (cell culture) excludes the potential influence of hormonal (angiotensin) or metabolic (calcium, phosphorus) changes in the observation. In cultured renal cells, both calcitriol and paricalcitol reduce expression of inflammatory mediators in response to high glucose concentrations, such as interleukin (IL) -6, monocyte chemotactic protein-1 (MCP-1) and IL-8 in podocytes and tubular cells.35
Antiproteinuric effect of vitamin D receptor activators in experimental nephropathies
Diabetic knockout mice for VDR develop higher albuminuria and glomerular sclerosis and have lower nephrin levels than the control mice.36 This observation indicates a renoprotective effect of endogenous vitamin D, without additional exogenous requirements, and may explain why the deficiency or insufficiency of vitamin D has a negative impact on proteinuria. Since the 1990’s, several studies have addressed the possible antiproteinuric effect of VDRA on experimental nephropathies. VDR activation may have an antiproteinuric effect on glomerular nephropathies of immune origin, subtotal nephrectomy CKD, diabetic nephropathy and puromycin nephrotic syndrome.
In 1993, the effect of vitamin D on the modulation of the immune system was explored, as a therapeutic approach in glomerulonephritis of immune origin. In rats with Heymann nephritis, a membranous nephropathy model, calcitriol at doses of 250-500ng/kg/48 hours significantly reduced proteinuria in 7-9 weeks, with no changes in antibody titres. However, calcitriol produced hypercalcaemia and increased calciuria.37
In rats with nephrotic syndrome induced by a toxin for podocytes (puromycin aminonucleoside) an increase was observed in the expression of 24-hydroxylase as well as a decrease in 1-α-hydroxylase that preceded the manifestation of renal failure. This pattern of gene expression led to a decrease in calcitriol. Furthermore, daily administration of calcitriol or its analogue 22-oxacalcitriol prevented podocyte lesion and at high doses, proteinuria was significantly suppressed, suggesting that the deterioration in vitamin D regulation plays an important role in the onset of proteinuria.26
The db/db mice, a genetic murine model of type 2 diabetes with less severe renal manifestations, overexpressed 23 genes related to vitamin D and there was an increase in 1-α-hydroxylase and glomerular VDR compared with non-diabetic db/+ mice. The authors hypothesised that the reduced severity of renal manifestations could be related to the expression of these protector genes, which increased even further in situations of hyperglycaemia. Higher expression of 1-α-hydroxylase prevented an increased production of fibronectin and type IV collagen induced by high concentrations of glucose in podocytes, which may explain the resistance of these mice to progressive diabetic nephropathy.38 In this regard, activation of VDR by calcitriol or paricalcitol prevented the profibrotic and proinflammatory response of podocytes to the presence in the microenvironment of another metabolic product whose accumulation causes proteinuric nephropathy, globotriaosylceramide accumulated in Fabry’s disease.39
In rats with subtotal nephrectomy, two different doses of paricalcitol (100 and 300ng/kg/3 times x week) for eight weeks decreased proteinuria and preserved kidney function independently of dose.40 In the same model, enalapril significantly improved proteinuria, and in the group with the combination of paricalcitol 800ng/kg three times per week and enalapril, proteinuria tended to decrease further and it decreased glomerulosclerosis and TGFβ1.41 and glomerulosclerosis decreased. In the same model, calcitriol 3ng/kg/day reduced albuminuria without significant changes in creatinine or calcaemia.42 To summarise, a very wide dose range, 21-2400ng/kg/week, had beneficial effects, suggesting that lower doses should be administered, which would presumably have fewer adverse effects. The human equivalent of these low doses is not very well known.
Another benefit of treatment with vitamin D analogues in animals is the suppression of renin, since the compensatory increase of renin is a physiological response to the use of ARBs as antiproteinuric agents. In mice with streptozotocin-induced diabetes, the combination of angiotensin 1 receptor blockers and vitamin D analogues completely prevented albuminuria, restored the glomerular filtration barrier structure and markedly reduced glomerulosclerosis. Paricalcitol prevented a decrease in nephrin mRNA and an increase in renin mRNA.43
Vitamin D also has anti-inflammatory effects. Local inflammation contributes to the progression of diabetic nephropathy.35,44 In rats with diabetic nephropathy, a dose of calcitriol or paricalcitol that did not reduce proteinuria, did however, produce anti-inflammatory effects, reducing renal expression of IL-6, MCP-1 and IL-8 mRNA in podocytes and tubular cells, as well as glomerular infiltration by macrophage.35
In summary, VDRA have an antiproteinuric effect in different animal models (Figure 1). In addition, there is a biological plausibility that explains antiproteinuric effects of VDR activation, either through renin suppression, regulation of inflammation/fibrosis and the direct effects on the podocyte or though antiapoptotic action, preservation of the slit diaphragm, anti-inflammatory and antifibrotic effects, which indicate that VDR activation may be an alternative for the treatment of proteinuria. However, from the clinical point of view, it is essential to confirm these potential benefits in clinical trials in patients, in order to answer the question asked by professor Cosio during the 2012 congress of the Spanish Society of Nephrology: is the rat lying to us?
ANTIPROTEINURIC EFFECT OF VITAMIN D. EVIDENCE IN CLINICAL RESEARCH
In clinical practice, it is useful to distinguish between indications approved for a drug and potential benefits suggested by experimental data that have not yet been validated for use in humans.
Vitamin D and selective vitamin D receptor activator indications according to data sheet
Initially, we will review the indications that are approved for these products on their data sheets. Natural or native vitamin D, cholecalciferol or vitamin D3 and ergocalciferol or vitamin D2 have therapeutic indications outside the kidney for the prevention and treatment of vitamin deficiency.
Calcifediol or calcidiol is 25 (OH) D3 and is indicated for the treatment of some forms of osteopenia, rickets or osteomalacia, hypophosphoraemia and, very generically, for renal osteodystrophy. VDRA have (rather outdated) indications for managing phosphocalcic disorders and renal hyperparathyroidism. The VDRA most used in Spain is calcitriol. sVDRA, namely paricalcitol, are indicated in the treatment of secondary hyperparathyroidism in renal patients. Potential beneficial pleiotropic effects of vitamin D derivatives are not yet included in the data sheet or in the clinical practice guidelines, as is the case for KDIGO.1,45
Scientific scales of evidence
Scales of evidence are the reference for providing the quality level of the studies and establishing the strength of recommendation. Currently, the scale most used is that developed by the Grade Working Group who use the KDIGO guidelines46,47 with slight modifications. According to these categories, the gold standard for reaching the first level of evidence is randomised clinical trials (RCTs) with sufficient statistical power and adequate sample size or systematic revisions, with their corresponding meta-analyses, that include RCTs and that are well designed.
However, the evidence hierarchy may increase or decrease the level in accordance with several considerations: a) the importance of the outcome, b) controlled design (placebo or reference standard) c) blinding, d) analysis of the sample size and follow-up time, e) statistical adjustment and assessment of conclusive statistics, etc. Therefore, overall analysis involves a certain amount of subjectivity, since there are no set rules for assessing these parameters. One example is the number of patients to be included: studies on few patients may artificially increase the magnitude of effect and induce a bias in the quality of evidence.
These prior considerations will assist us in assessing the level of evidence and strength of recommendation for the antiproteinuric effect of vitamin D and its derivatives in the clinical field.
Antiproteinuric effect: clinical information available
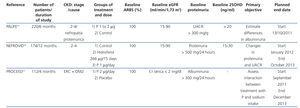
Table 1 displays the main RCTs that have explored the effect of vitamin D and VDRA on proteinuria, and Table 2 displays the studies currently underway.
- Native vitamin D. The determination of serum 25(OH)D3 is the standard test for assessing vitamin D levels. In a broad cross section of the population (NHANES III) an inverse relationship was found between levels of 25(OH)D3 and a higher prevalence of albuminuria.48 However, we did not find intervention studies that explore the antiproteinuric effect of native vitamin D.
- Calcitriol. Liu et al.49 showed in an RCT of 50 patients with IgA nephropathy and residual proteinuria >0.8g/day that patients receiving 0.5μg calcitriol twice weekly had a mean decrease in proteinuria of 19% (mean of 1.6 to 1.3g/24 hours) compared with placebo, after 48 weeks of follow-up.
Analysis: small sample size, unblinded, without clinically relevant data, such as blood pressure. The decrease in proteinuria, although significant, was small (1.6 to 1.3g/24 hours) and there was no change in the glomerular filtration rate. Although it is a study suggestive of the antiproteinuric effect of calcitriol, the quality of evidence and strength of recommendation are low. Furthermore, it is a single test (requiring verification), with results that cannot be extrapolated to other kidney diseases.
- Paricalcitol. Four studies and a meta-analysis evaluated the antiproteinuric effect of oral paricalcitol in patients with stages 2 to 4 (S2-4) CKD. The four studies analysed residual proteinuria in patients previously treated with ARBs (Table 1).
Agarwal et al.16 observed in an RCT conducted in patients with residual proteinuria and S2-4 CKD a decrease in proteinuria (dipstick) in 52% of patients treated with paricalcitol compared with 27% in the control group (p=.025) after 24 weeks of treatment.
The authors concluded that the antiproteinuric effect of paricalcitol is a potential pharmacological action that requires further investigation.
Analysis: This study was designed to assess the suppression of the parathyroid hormone, with antiproteinuric effect being a post hoc analysis. Therefore, the quality of evidence is low. Furthermore, the sample was relatively small and the assessment of proteinuria was semi-quantitative, with no values for proteinuria. There were no changes in renal function.
Alborzi et al.17conducted a trial designed for three groups of patients with S2-4 CKD: no treatment, 1μg paricalcitol and 2μg paricalcitol. The trial included 8 patients in each group and the follow-up time was one month. The study variables were albuminuria, inflammation, blood pressure and endothelial function.
Analysis: it could be considered a pilot study, given the small sample size and short follow-up period. The decrease in albuminuria was analysed only in percentage terms, and although it was close to 50% in the paricalcitol group, the small sample size prevented extraction of information that was capable of establishing the quality of the evidence. In addition, baseline albuminuria was four times higher in the control group than in the paricalcitol group.
Fishbane et al.18 also published an RCT comparing paricalcitol (n = 28) with placebo (n = 21) in patients with S2-3 CKD, over a follow-up period of six months. The decrease in the urine protein to creatinine ratio was 2.8 compared with 2.3g/day (17.6%), p=.04; 57% achieved a decrease greater than 10% (compared with 30% in the placebo group).
Analysis: RCT conducted on a very small number of patients, with an unclear estimated sample size, low statistical power and heterogeneity in the results. The modest reduction in proteinuria and the absence of data on the progression of CKD limit the value of the drug’s antiproteinuric effect.
We can conclude that these three studies are an important aid for recommending the study the antiproteinuric effect of paricalcitol, but the quality of evidence and strength of recommendation are insufficient to support changes in clinical practice. Furthermore, the inclusion of patients deficient in vitamin D prevents distinction between the intrinsic therapeutic effects of paricalcitol and the effects of correcting the vitamin D defect.
De Zeeuw et al.19 published an RCT specifically designed to assess in type 2 diabetic patients with a glomerular filtration rate between 15 and 90ml/min and residual albuminuria the antiproteinuric effect of paricalcitol in doses of 1 and 2μg. The first target was the percentage change in the urinary albumin to creatinine ratio (ACR), which did not reach statistical significance. In groups with paricalcitol, albuminuria decreased by 16% and 20%, respectively. The mean decrease in 24-hour albuminuria was 254mg (from 717mg to 463mg, 34%) in the 2μg paricalcitol group. The percentage of patients with a drop in the ACR >15% only achieved minimum significance (P=.038) in those who received 2μg of paricalcitol. It is notable that after six months of treatment, a 5% decrease in the estimated glomerular filtration rate was found in the group treated with 2μg of paricalcitol. A post hoc analysis identified patients treated with 2μg/day and high intake of salt as the only patients who could benefit from treatment. However, in 42% of these patients, it was necessary to reduce the dose of paricalcitol and the incidence of dialysis or death was 6-7 times higher in the 2μg paricalcitol group than in the placebo group (p=.064-.118, we cannot be more specific due to a lack of sufficient published data).
Analysis: this is a well-designed, blinded, controlled RCT with an adequately estimated sample size. Neither the ACR (surrogate parameter) or renal function showed a clear benefit from the intervention. The first outcome, which was the change in the ACR with paricalcitol compared with placebo, was not significant. The variability of the ACR was not considered, which further decreases the statistical power. The mean decrease in albuminuria was subtle, less than 250mg/24 hours, and always starting from relatively low mean baseline values (<0.8g/24 hours). The percentage of patients with a decrease in the ACR greater than 15% was significantly higher in patients who received paricalcitol (p=.038). Also, patients who received paricalcitol showed a slight and transient decrease in their estimated glomerular filtration rate. These results do not conclusively explain the potential antiproteinuric benefits of paricalcitol. Also, prior vitamin D repletion (in a highly deficient population) and the comparison with other cheaper active forms of vitamin D were not considered. Again, the inclusion of patients deficient in vitamin D (25-OHD mean 16.6ng/ml)50 prevents distinction between the intrinsic therapeutic effects of paricalcitol and the effects of correcting the vitamin D defect.51
Cheng et al.52 published a meta-analysis that evaluated the effect of paricalcitol on hyperparathyroidism and residual proteinuria (post-ARB) in patients with S2-5 CKD. We selected the four aforementioned studies. Given the lack of data for assessing final proteinuria, the decrease in proteinuria>10% was applied as an end-point or result. We must confirm that the method of measuring this variable was not uniform in all four studies (dipstick, albuminuria, urine protein to creatinine ratio and ACR, respectively). From the four RCTs assessed, a total 469 patients were recruited: 285 treated with paricalcitol and 184 with placebo. The overall analysis showed a significant benefit in reducing proteinuria >10% (relative risk [confidence interval 95%]: 1.68 [1.25-2.25]). This meta-analysis has major limitations; the greater statistical power is provided by the RCTs with smaller series and a more questionable design. In fact, trials with small series usually show greater therapeutic effects by accentuating the differences in case selection. Likewise, when only patients treated with 2μg paricalcitol were considered, there were no significant treatment benefits.
CONCLUSIONS
There is experimental evidence that VDR activation exercises a protective action on the podocyte, limits activation of the renin-angiotensin-aldosterone system, slowing renin production and has an antiproteinuric effect. However, the latter effect has been observed with highly variable doses of VDRA, which begs question, what is the optimal dose and can the mere substitution of a vitamin deficiency useful? Clinical trials have been conducted generally in patients with an overall deficiency or insufficiency of vitamin D, which does not help clarify this issue. Moreover, these tests showed poor tolerance to higher doses of paricalcitol (2µg/day). There is evidence to suggest that animals in experiments and humans may respond differently to VDR activation. Thus, paricalcitol did not decrease plasma renin activity in the VITAL study19 or avoid a remarkable hyperplasia of the renin-producing juxtaglomerular apparatus in a patient with a renal biopsy after proteinuria treatment.53
In summary, the information available is insufficient for us to advise the use of native vitamin D, VDRA or sVDRA as renoprotective antiproteinuric drugs beyond the experimental level. Moreover, these data cannot clarify whether the magnitude of decrease in proteinuria achieved in the studies (>10% in this meta-analysis) has an impact on the progression of CKD. Further studies should establish within what ranges of proteinuria real efficacy is obtained. Likewise, cost-effectiveness studies will help defend the indication. We estimate that the dialysis treatment may increase the annual cost of the patient by about 40,000 euros.54 Any therapeutic measure that delays the start of dialysis for a mean of six months, for instance, could be effective if its weekly cost were less than this figure. In this sense, all VDRA, including paricalcitol, have a significantly lower cost.
KEY CONCEPTS
- Proteinuria is the main predictor of CKD progression. After using ARBs, residual proteinuria continues to be a priority therapeutic target.
- Several experimental models suggest an antiproteinuric effect of VDR activation due to various mechanisms: renin suppression, regulation of inflammation, direct effects on the podocytes and antifibrotic effects.
- From the clinical point of view, the information available is insufficient to advise the use of native vitamin D or VDRA as additional renoprotective antiproteinuric drugs beyond the experimental setting.
Acknowledgements
Instituto de Salud Carlos III (ISCIIIRETIC REDINREN RD12/0021, PS09/00447), Comunidad de Madrid (CIFRA S2010/BMD-2378), Programme to Encourage Research (Programa Intensificación Actividad Investigadora) (ISCIII/Agencia Laín-Entralgo/CM) to AO.
Conflicts of interest
VLS was the main researcher in the NEFROVID study and AOA is a member of the management committee in the PALIFE study.
Table 1. Main published randomised clinical trials that explore the effect of paricalcitol on proteinuria
Table 2. Clinical trials currently being carried out that explore the effect of paricalcitol on proteinuria
Figure 1. Mechanisms potentially involved in the antiproteinuric and renoprotective effect of vitamin D receptor activation