Introducción: La vitamina D posee un efecto regulatorio del eje renina-angiotensina-aldosterona, jugando, por lo tanto, un papel importante en cuanto a proteinuria se refiere. Presentamos nuestra experiencia en el uso de paricalcitol como antiproteinúrico. Métodos: Incluimos 36 pacientes con un eGFR of 30-90 ml/min/1,73 m2 y proteinuria > 400 mg/d con dosis estables de inhibidores del SRAA durante 3 meses. Se le admistró durante 12 meses 1 µg/día de paricalcitol. Como objetivo primario estudiamos el descenso de proteinuria; como secundarios cambios en Cr, eFG, calcio, fósforo, iPTH, 25(OH)vitD, PCR y tension arterial. Resultados: La proteinuria media fue 2806 mg/d cayendo hasta 2199 mg/d en el mes 6 (p < 0,0001) y 1931,5 mg/d a los 12 meses (p < 0,0001). Aquellos con una proteinuria basal > 3000 mg/d (n=12) sufrieron una menor disminución (5956,9 ± 2492,6 mg/d a 4220,4 ± 2613 mg/d en mes 12) respecto a aquellos con una proteinuria < 3000 mg/d (1371 ± 627,5 mg/d a 821,3 ± 491,5 mg/d en mes 12). No se objetivaron cambios en tension arterial, eGFR y PCR. Los cambios en calcio, fósforo, iPTH y vitamina D 25(OH) fueron estadísticamente significativos. Conclusión: Nuestro estudio demuestra una reducción importante de proteinuria con dosis bajas de paricalcitol en pacientes con IRC, que es de particular importancia en aquellos con porteinuria basal entre 1-3 g/d.
Background: Vitamin D has an important regulatory effect on the renin-angiotensin-aldosterone system, playing a central role in the regulation of proteinuria. We therefore studied the antiproteinuric effect of paricalcitol. Methods: 36 patients with an estimated GFR of 30-90mL/min/1.73m2 and proteinuria >400mg/d with a stable dose of ACE inhibitor or ARB for at least 3 months were recruited. Patients received oral paricalcitol 1µg/day for 12 months. Primary endpoint was decrease in proteinuria from baseline. Secondary endpoints were changes in creatinine, eGFR, serum levels of calcium, phosphorus, iPTH, 25(OH)vitD, C-Reactive Protein and blood presure. Results: Mean proteinuria was 2806mg/d and fell to 2199mg/d at month 6 (p<.0001) and 1931.5mg/d at month 12 (p<.0001). Patients with >3000mg/d baseline proteinuria (n=12) saw smaller relative reductions in proteinuria (5956.9±2492.6mg/d to 4220.4±2613mg/d at 12 months) than patients with <3000mg/d baseline proteinuria (1371±627.5 mg/d to 821.3±491.5mg/d at 12 months). There were no changes in BP, eGFR and CRP. We observed significant changes in serum levels of calcium, phosphorus, iPTH, 25(OH) vitamin D. Conclusion: Our study shows an important reduction in proteinuria with a low dose of oral paricalcitol in CKD, that is particularly robust with baseline proteinuria between 1-3g/d.
INTRODUCTION
Proteinuria is a marker of chronic kidney disease (CKD) progression and a predictor of cardiovascular disease and death.1 This is particularly true in diabetic nephropathy, but also applies to other proteinuric disorders such as idiopathic glomerular diseases.2
Active vitamin D (AVD) deficiency, is especially pronounced in CKD, even in its earliest stages, and it has been identified in clinical and experimental studies as a novel risk factor for progression of kidney and cardiovascular disease.
Current evidence suggest that 1,25-dihydroxyvitamin D3 and its analogs inhibit RAAS by supressing renin gene transcription;3 regulate cell proliferation, apoptosis, and angiogenesis; and have anti-inflammatory effects.4-7 Recent short-term studies in humans have suggested that activated vitamin D treatment may reduce proteinuria.8-11 In this study, we evaluated the effect of paricalcitol on proteinuria reduction in CKD patients over a longer period of 12 months.
SUBJECTS AND METHODS
Study Participants
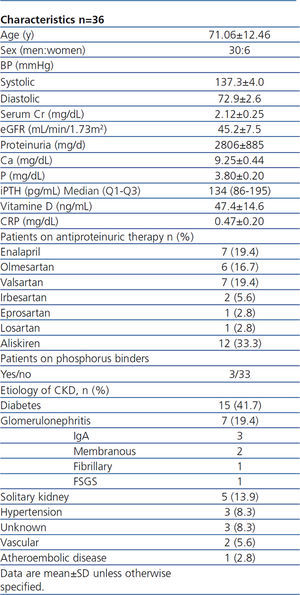
In this open-label single-arm study, we recruited patients from the renal clinics at Hospital Universitario de La Princesa (Madrid, Spain). Eligible patients were ≥18 years of age, with an eGFR of 30-90mL/min/1.73m2 (calculated by using the 4-variable Modification of Diet in Renal Disease [MDRD] Study equation), and persistent proteinuria >400mg/d with a stable dose of ACE inhibitor, renin inhibitor or ARB for at least 3 months. Patients were required to have intact parathyroid hormone (iPTH) 70pg/mL-270pg/mL, with serum 25(OH)vitaminD >30ng/mL without being treated before with any form of vitamin D. We excluded those with serum phosphorus (P) >4mg/dL, serum calcium (Ca) (adjusted for albumin) >10.0mg/dL, uncontrolled hypertension, and glomerulonephritis (GN) requiring immunosuppresive therapy. Written informed consent was obtained from all patients.
Patients received oral paricalcitol 1µg/d and were followed for 12 months. Baseline measurements were done in the first visit, with a safety consultation 4 weeks after starting the medication for measurement of Ca, P, and creatinine (sCr). At months 6 and 12, blood tests and urinaliysis were performed, measuring 24 hour urine protein, eGFR, iPTH, Ca, P, sCr, 25(OH)vitamin D, C-Reactive Protein (CRP).
The dose of paricalcitol was adjusted based on iPTH level, Ca or P. If iPTH was <70pg/mL, the dose was reduced by 50% (1µg/48hours); if iPTH >270pg/mL, the dose was increased to 2µg/d; if iPTH >400pg/mL, the patient would be removed from the study. Adjustment through Ca ocurred in 3 patients with hypercalcemia >10mg/dl with a subsequent dose reduction of 50%. Adjustment based on serum P was not necessary. After every change of dose, a new visit in 4 weeks was scheduled. During the study period we aimed to continue the usual optimal dosage of all concurrent medications, particularly RAAS blocking therapy.
End Points
The primary endpoint was decrease in urine protein excretion from baseline. Secondary endpoints included occurrence of side efects and changes in Ca, P, iPTH, 25(OH)vitamin D, CRP, sCr, eGFR and BP between first and last visit of follow up.
Statistical Analysis
Continuous variables are reported as mean ± SD, except iPTH expressed as median (Q1-Q3). Categorical variables are reported as frequency. We analyzed the differences in proteinuria(mg/d), serum levels of Ca(mg/dL), P(mg/dL), iPTH(pg/mL), 25(OH)vitamin D(ng/dL), CRP(mg/dL), and eGFR betwen visits at 0, 6 and 12 month using Student’s T- test for paired comparison. An intra-group analysis of proteinuria as a categorical variable (>3g/d, <3g/d, <1g/d, 1-3g/d and >3g/d) was done between mentioned visits using Stuart-Maxwell test. Proteinuria reduction was compared between different RAAS blockers using Kruskall-Wallis test. Multiple comparisions were performed using Wilcoxon-Rank Sum test. Between-groups analysis of proteinuria vs. etiology was done using Kruskall-Wallis test and multiple comparisions through Wilcoxon-Rank Sum test. Multivariate analysis was performed for age, gender and type of antihypertensive drug.
RESULTS
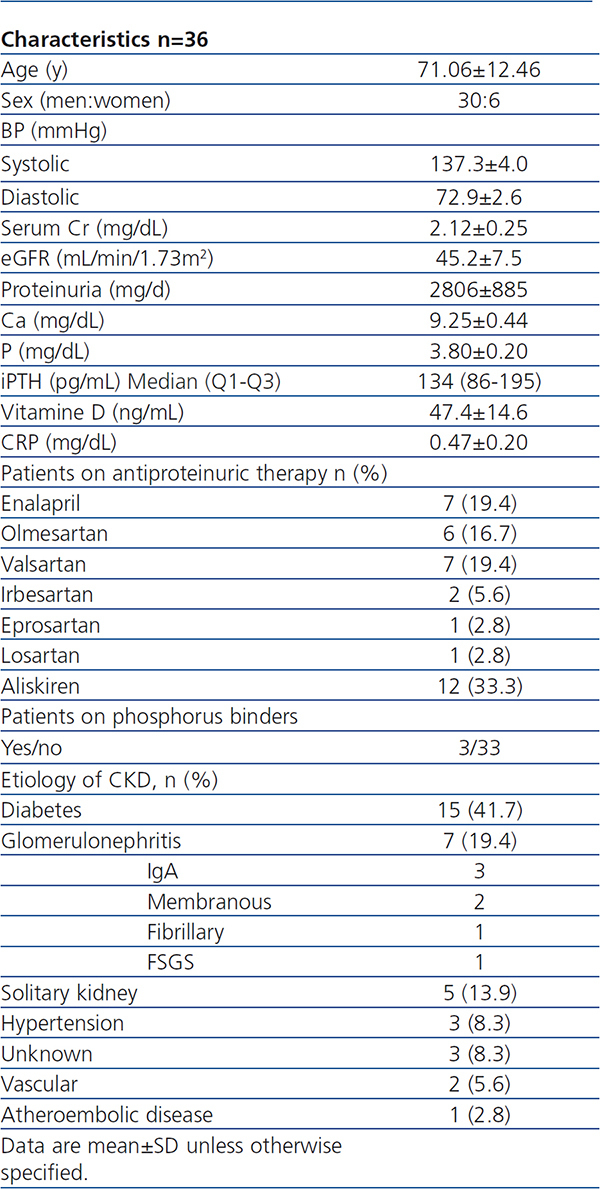
A total of 36 patients were included and completed the study. Baseline clinical characteristics of these patients are listed in Table 1. No patient needed additional medications to control BP during the study. All participants were treated with only one RAAS blocking drug at maximal tolerated dose. Only 3 patients were treated with P binders (non-calcium) before the study. Just 3 patients required dose changes of paricalcitol; this was due to mild hypercalcemia prompting a reduction to 1µg/48h. No other adverse effects occurred.
Proteinuria and Renal Function
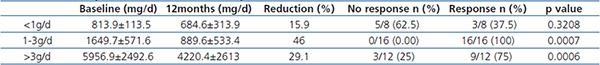
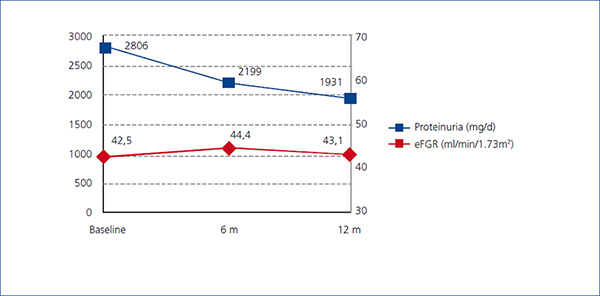
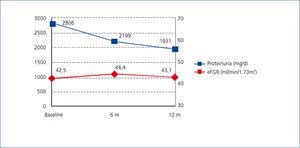
There was a significant decrease in proteinuria between baseline and visits at 6 and 12 months. Mean proteinuria at baseline (2806mg/d) was significantly higher (p<0.0001) than the value at 6 months (2199mg/d) and at 12 months (1931.5mg/d) (p<0.0001). Overall, mean proteinuria was reduced by 31.2% at the end of the study.
Patients post-hoc were analyzed based on baseline proteinuria (Table 2). The decrease in protenuria in patients with <3g/d was significant, falling from 1371±627.5mg/d at baseline to 821.3±491.5mg/d at month 12, a 40.1% reduction from baseline (p<0.0001). Among patients with >3g/d (mean 5956.9±2492.6mg/d), proteinuria fell by 29.1% to 4220.4±2613mg/d after 12 months of paricalcitol treatment (p=0.0006). In further subgroup classification, those with baseline proteinuria between 1-3g/d demonstrated a 46% at 12 months (p=0.0007).
Additionally, no differences in proteinuria decline were observed between the different causes of CKD. Changes in proteinuria were not associated with changes in eGFR, which remained stable throughout the study (Figure 1).
It is important to notice that patients with diagnose of GN were not requiring immunosuppresive therapy. This subgroup of patients reached a reduction of 35.6% from baseline.
Blood Presure and Laboratory Parameters
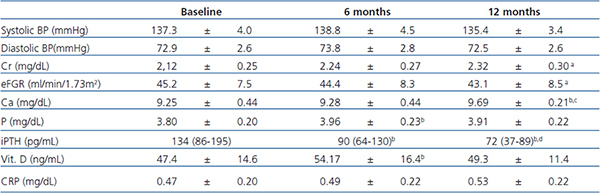
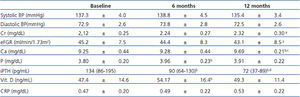
Mean systolic and diastolic BP remained relatively constant throughout the course of study. Every subject received only one antihypertensive drug, all of them RAAS blocking therapy, and doses did not undergo a significant change along the study. Despite BP mesures at clinic, home BP was always below the target for proteinuric nephropaties.
CRP was measured as a marker of inflammation, no significant diferences were observed at months 6 and 12. Ca, P, iPTH, and 25(OH)vitaminD did change significantly during the study period. Ca rose from mean baseline 9.25±0.44mg/dL to 9.69±0.21mg/dL at month 12 (p<0.05). Mild hypercalcemia (i.e.<11.2mg/dL) occurred in 3 subjects four weeks after starting paricalcitol treatment (not due to P binders). The dose of paricalcitol was reduced 50% and another laboratory test was done in 4 weeks without abnormal findings. Phosphorus also increased significantly but still remained in normal range. As expected, iPTH fell at month 6 (p=0.04) and at month 12 (p<0.0001). Levels of 25(OH)vitaminD were significantly increased at month 6 compared with baseline (p=0.048), but there were no differences between baseline and final visit. (Table 3).
Multivariate analysis was performed for age, gender and type of antihypertensive drug. Only age reached statistical signification (p=0.046), subjects <70 years had greater reduction compare to those with >70.
DISCUSSION
Here we present the results of 12 months of treatment with oral paricalcitol on proteinuria. These results here represent, to date, the longest published experience of using this specific formulation of AVD as an anti-proteinuric therapy. In our study we observed that daily treatment with oral paricalcitol at 1µg/d produced a significant decrease in proteinuria in patients with CKD already on maximally tolerated doses of RAAS blockade. Side effects were rare and limited to mild hypercalcemia, which was quickly reversed with dose reduction.
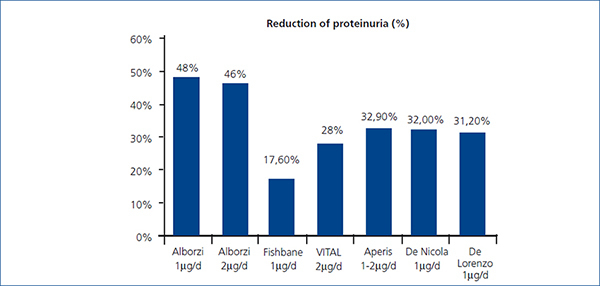
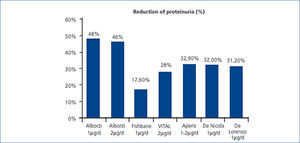
Our findings agree with those of other previously reported studies. Agarwal et al., in 3 double-blind, randomized, placebo-controlled studies, demonstrated that paricalcitol with a mean dose of 9.5µg/week p.o (24 weeks) reduced proteinuria in 29/57 (51%) patients with CKD stages 3 and 4.9 They reported odds for reduction in proteinuria 3.2 times greater for paricalcitol patients compared to placebo patients. Alborzi et al. published a study with 24 patients allocated to 3 groups to receive, for 1 month, 0, 1, or 2µg of oral paricalcitol. The placebo group had 1.35 times the baseline urinary albumin excretion (UAE), whereas the 1µg group had 0.52 times and 2µg had 0.54 times the baseline UAE. Reductions in CRP suggest that the anti-proteinuric effect could be explained by antinflamatory effect.11 Fishbane et al. conduced a double-blind, randomized, placebo vs. paricalcitol (1µg/d) study with 61 patients treated for 6 months. In paricalcitol group, there was a significant decrease of proteinuria mesured by uPCR of -17.6%±47.8% compared with controls +2.9%±19.0% (Figure 2).10 Aperis et al. demonstrated an average 32.9% reduction of proteinuria in 19 patients treated with 1-2μg daily paricalcitol with 74% of respond, specially in diabetic nephropathy.12 In VITAL study 281 type 2 diabetic patients with albuminuria, were randomly allocated to receive for 24 weeks placebo, 1µg or 2µg/d of paricalcitol, in addition to ACEIs or ARBs. A significant decrease of proteinuria was reported. In the combined paricalcitol groups the reduction was 16%. With 1µg/d, proteinuria fell by 14% and 20% with 2µg/d. They evidenced a decrease in BP but not in CRP. This study suggests that a dose of 2μg/day of paricalcitol in diabetic nephropathy, added to RAAS inhibition, lowers residual albuminuria.13 Our study showed a significant decrease of 26.9% in proteinuria in the subgroup of diabetic patients. Our results are as well similar to the latest published by De Nicola et al (32% reduction, p<0,05) and Blanco-García et al (27% of reduction, p=0,1) both with 6 months follow up period.14,15
In our study, we demonstrated a substantial proteinuria reduction in 78% of patients, and the mean proteinuria decrease in our patients was higher than those reported by Fishbane et al. and VITAL study, but similar to those reported by Alborzi, Aperis and De Nicola. Our results are unique in the duration of treatment and follow-up (12 months), suggesting a sustained benefit of AVD therapy. We speculate that we saw a greater reduction in proteinuria than Fishbane et al. and VITAL, despite using the same dose of drug and treating patients with nearly equal mean baseline proteinuria, due to the longer treatment period in our study.
The results of our subgroups also shed light, potentially, on which group of patients may expect to see the most benefit from vitamin D, as 100% and 75% of patients with 1-3g/d and >3g/d proteinuria, respectively, responded to paricalcitol, while those with <1g/d had a far lower response rate. As with previous studies of vitamin D therapy as an anti-proteinuric agent, we saw no changes in BP or eGFR to explain the reduction. The drug appears to work as an anti-proteinuric via its anti-inflammatory properties, although we did not demonstrate a significant reduction in CRP; theoretically those patients with very low proteinuria (i.e. <1g/d) likely have far less inflammation in the pathogenesis of their proteinuria.
The anti-proteinuric effect of vitamin D can also be explained by its important regulatory effect on the RAAS, which in turn has a central role in the regulation of BP and UAE. Li et al. demonstrated that renin production was increased in vitamin D receptor mutant mice and it was independent of Ca or PTH.16 Furthermore, 1,25-Dihydoroxyvitamin D3 suppresses renin gene transcription.3 In experimental models, rats with 5/6 nephrectomy, paricalcitol decrease angiotensinogen, angiotensin type 1 receptor, renin, renin receptor, and VEGF.17 The down regulation of RAAS achieved by vitamin D or its analogues should therefore yield beneficial effects, in light of clinical studies that have demonstrated that RAAS inhibition slows the progression to ESRD in proteinuric CKD.18-20
Our study has limitations, however. The lack of a control group makes it difficult to ascribe the proteinuria reduction solely to vitamin D therapy, although we note that previous studies which did use a placebo arm achieved very similar results as ours. The incidence of hypercalcemia as an adverse effect of therapy was low (8.3%) but may accumulate over time; therefore, the low-risk profile of this therapy can only be claimed for the 12 months of therapy reported here. Finally, our main outcome, proteinuria, remains a surrogate outcome for the far more important outcomes of declining eGFR, ESRD, cardiovascular disease, and death. We can only speculate that the beneficial effects of vitamin D therapy on proteinuria will extend to significant alterations in these hard outcomes. Future studies will need to treat (and follow) patients for longer periods of time to evaluate these issues.
CONCLUSION
Our study is the first to use oral paricalctol for 1 year specifically as an anti-proteinuric therapy. Our results confirm the findings of previous shorter-term studies and promote the use of AVD as a long-term anti-proteinuric agent to complement long-term use of RAAS blockade. This therapeutic strategy may be particulary beneficial for those patients with >1g/d proteinuria.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Baseline Characteristics
Table 2. Percent of proteinuria reduction and response
Table 3. Blood presure and serum measurements
Figure 1. Modification of proteinuria and eGFR
Figure 2. Comparision of proteinuria reduction