KDRI/KDPI are tools use in kidney donor evaluation. It has been proposed as a substitute of, or complementary to preimplantation renal biopsy. These scores have not been validated in Spain.
Objective(1) To investigate the concordance between KDPI and histological scores (preimplantation renal biopsy) and (2) to assess the relationship between KDRI, KDPI and histological score on graft survival in the expanded criteria donors group.
MethodologyRetrospective cohort study from 1 January 1998 to 31 December 2010.
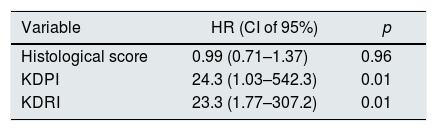
ResultsDuring the study 120 donors were recruited, that resulted in 220 preimplantation renal biopsies. 144 (65%) grafts were considered suitable for kidney transplantation. 76 (34.5%) were discarded. Median follow up has been 6.4 years (sd 3.9). Median age 63.1 years (sd 8.2), males (145; 65.9%), non-diabetic (191; 86.8%) and without another cardiovascular risk factors (173; 78.6%). 153 (69.5%) donors died of cerebrovascular disease. There were significant differences in KDRI/KDPI score in both groups 1.56/89 (sd 0.22) vs 1.66/93 (sd 0.15), p<0.01. The KDPI showed moderate concordance and correlation with the histological score (AUC 0.64/correlation coefficient 0.24, p<0.01). KDPI (HR 24.3, p<0.01) and KDRI (HR 23.3, p<0.01) scores were associated with graft survival in multivariate analysis.
Conclusion(1) KPDI and histological scores show moderate concordance. The utility of both scores as combined tools it has to be determined. (2) KDPI score, and especially KDRI score, are valid for estimating graft survival and combined with the biopsy can help to individualized decision making in the expanded criteria donors pool.
El KDRI y su variante KDPI son dos herramientas utilizadas para la valoración del donante renal. Se ha propuesto la utilidad del KDPI como sustituto/complementario a la biopsia renal preimplantación. Estos scores no están validados en España.
Objetivo1) Investigar la concordancia entre los scores KDPI e histológico (biopsia renal preimplantación), y 2) valorar la relación entre el KDRI, KDPI y la puntuación histológica sobre la supervivencia del injerto, en donantes con criterios expandidos (ECD).
MetodologíaEstudio de cohortes, unicéntrico, retrospectivo desde el 1 de enero de 1998 hasta el 31 de diciembre de 2010.
ResultadosSe reclutaron 120 donantes y 220 biopsias preimplantación. Ciento cuarenta y cuatro (65,5%) injertos fueron aptos para trasplante. Setenta y seis (34,5%) fueron descartados. Tiempo medio de seguimiento 6,4 años (ds 3,9). Edad media de los donantes 63,1 años (ds 8,2), varones (145; 65,9%), no diabéticos (191; 86,8%) y sin otros factores de riesgo cardiovascular (173; 78,6%). Causa de muerte mayoritaria ACV hemorrágico (153; 69,5%). La puntuación KDPI media entre los grupos riñón válido (1,56/89; ds 0,22) y no válido (1,66/93; ds 0,15) es estadísticamente significativa (p < 0,01). El KDPI mostró una concordancia y correlación moderadas con el score histológico (AUC 0,64/coeficiente de correlación 0,24, p < 0,01). Los scores KDPI (HR 24,3, p < 0,01) y KDRI (HR 23,3, p < 0,01) están relacionados con la supervivencia del injerto en el análisis multivariante.
Conclusión1) Los scores KDPI e histológico presentan una concordancia moderada. 2) Las puntuaciones KDPI, y sobre todo KDRI, son válidas para estimar la supervivencia de los injertos y pueden ser utilizadas de forma combinada con la biopsia para la toma de decisiones individualizadas en el grupo de donantes con criterios expandidos.
Kidney transplant from donors with expanded criteria (ECD) is under debate for two reasons: (1) the imbalance between the available organs and patients on the waiting list for kidney transplantation and (2) the changes experienced in the donor's profile. Hemorrhagic or ischemic stroke has become the leading cause of death, this is associated to an increase in both the mean age and the risk factors of cadaveric donors.1 Using the UNOS 20012 criteria for the selection of donors, more than 50% of the donors generated annually in Spain would be labeled as ECD donors, which sometimes requires preimplant biopsy in order to assess the viability of the graft.
In the last decade, several scales have been developed to measure the prognosis of the graft trying to eliminate the dichotomy of standard donor (SCD) versus the ECD donor. The idea is to perform an individualized assessment of graft quality and graft survival, considering the characteristics of both donor and recipient. None of these scales has been validated for prediction of graft function in ECD donors in the different countries.3 The Kidney donor risk index (KDRI)4 and its adaptation to the Kidney donor risk profile (KDPI)5 have been developed by the American Registry of Transplants. The KDRI calculates the relative risk of graft failure and the grades are from 0.5 and 3.5. The KDPI score are from 0 to 100 points with a table of assigned values to the score obtained from the donor parameters of the KDRI score, in which a score of 85 means that 85% of the donors are of better quality. Both scores have been associated to the survival of the transplanted organ.
In Spain, ECD grafts are evaluated by preimplantation biopsy. In Andalusia, the biopsy is performed as part of the transplant protocol in patients with UNOS criteria and it is evaluated following the Andalusian protocol for the evaluation of renal biopsy.6 Currently there is no validated clinical tool to make decision beyond the UNOS 2001 criteria. However, recently the KDPI score has been proposed as an alternative to preimplantation renal biopsy.
Objectives of the present study are:
- 1.
To evaluate the correlation between the KDPI and histological scores (preimplantation renal biopsy) in ECD donors.
- 2.
To assess the relationship between KDRI, KDPI, histological score and graft survival.
This is a retrospective cohort including all the ECDs that underwent preimplant biopsy from January 1, 1998 to December 31, 2010 in the Granada-Jaén area, which provides health care to a population of 1,593,710 and performed approximately 60 cadaveric donor kidney transplants per year.
DefinitionsThe biopsies were performed by renal wedge, according to the previously published quality requirements7 the samples were fixed in Glyofix® (Pacisa-Giralt, Barcelona, Spain) and processed in the Pathology Department of the University Complex of Granada using the accelerated inclusion method in paraffin by microwave oven, according to standard procedure, and they were stained with hematoxylin–eosin and periodic acid Schiff stain. The histological evaluation was carried out according to the Andalusian preimplantation renal biopsy assessment protocol.7 The calculation of the score was based on the percentage of glomerular sclerosis determined quantitatively and, in a semiquantitative manner: tubular atrophy, neointimal arterial thickness and interstitial fibrosis. A sample was adequate if it contained at least 25 glomeruli and 2 small arteries. A score ≤7 was considered a favorable histology for transplantation.
The KDRI score was calculated according to the formula provided by Rao et al.4 The KDPI score was calculated using the formula available on the website of the Organ Procurement and Transplantation Network (OPTN/UNOS).8 The KDPI score is obtained from a table that assigns a percentage to a range of values resulting from the donor data of the KDRI score formula.9 For example, a score between 1.443571 and 1.466165 corresponds to a KDPI of 85%. The score resulting from the donor data of the KDRI score was used to perform statistical analysis; the percentage (KDPI) obtained with the extrapolation table was not used for statistical analysis. This approach eliminates the loss of information that would generate the grouping of donor score values in intervals to assign a percentage value. In the different sections, the KDPI values are expressed as number of points and percentage (in parentheses).
The creatinine clearance from donors was estimated by the Cockroft–Gault formula.
The delayed function of the graft was defined as the need for dialysis in the immediate post-transplant period.
Statistic analysisThe statistical analysis was performed using the PSPP4-GNU GPL V3 package and the r-comander UCA package. Normal distribution was tested by The Shapiro–Wilk test, or the Omnibus test by D’Agostino-Pearson. Differences between means was analyzed by Student t test or ANOVA, after analysis of the established conditions of application. The Chi-square test was used to compare proportions. The correlation between the histological score and the KDPI and KDRI scores was made using the Spearman correlation coefficient and the ROC curve. The survival analysis was estimated using the Kaplan–Meier method and the statistical significance between the survival times was determined with the log-rank test. The multivariate survival analysis was performed by Cox proportional hazards to identify the variables that influence overall survival. The level of statistical significance was p<0.05.
ResultsCharacteristics of the study populationDuring the study period there were 120 donors in whom a total of 220 biopsies were performed before transplantantation.
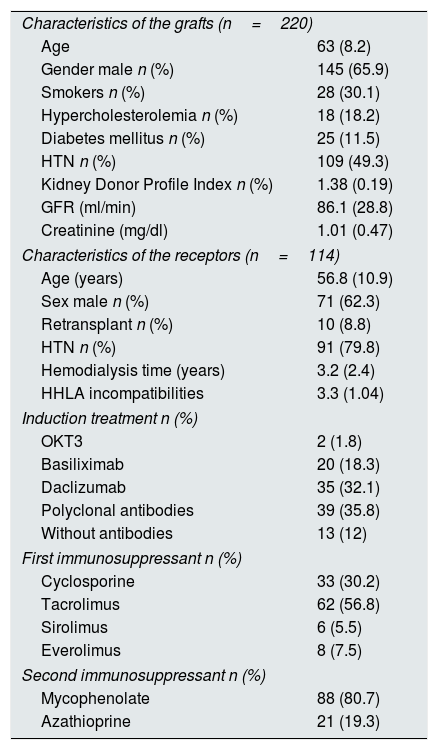
Of the total renal grafts, 144 were considered suitable for transplantation (65.5%) and 76 were considered non-appropriate for transplantation (34.5%). The correlation between the KDPI and histological scores was evaluated in 197 grafts, since in the remaining 23 it was not possible to calculate the KDPI score due to the lack of items that needed to be scored. Of the 144 eligible grafts, 114 were transplanted. Another 30 grafts were not implanted due to anatomical or extraction problems, or they were transplanted in another province and the graft survival could not be evaluated so they were eliminated from the analysis. The average follow-up time was 6.4 years (SD 3.9). The mean age of the donors was 63.1 years (SD 8.2), there were mostly males (n=145, 67.1%), non-diabetic (191, 86.8%) and without other cardiovascular risk factors (173, 80.8%). The main cause of death of ECD donor was cerebrovascular disease (172, 78.2%), with a predominance of hemorrhagic stroke (153, 70.2%). The mean age of the recipients was 56.8 years (SD 10.9), with a mean dialysis vintage of 3.2 years (SD 2.4). The immunosuppressant treatment mainly included prednisone, calcineurin inhibitors (cyclosporine or tacrolimus) and mycophenolate mofetil. The distribution of immunosuppressive treatment is presented in Table 1. A total of 107 patients (93.8%) were on steroids throughout the study period. The percentages of delayed graft function and acute rejection were 51.75% (59 patients) and 15% (17 patients), respectively.
Characteristics of the study population. The data are expressed in number and percentage or mean and standard deviation (SD).
| Characteristics of the grafts (n=220) | |
| Age | 63 (8.2) |
| Gender male n (%) | 145 (65.9) |
| Smokers n (%) | 28 (30.1) |
| Hypercholesterolemia n (%) | 18 (18.2) |
| Diabetes mellitus n (%) | 25 (11.5) |
| HTN n (%) | 109 (49.3) |
| Kidney Donor Profile Index n (%) | 1.38 (0.19) |
| GFR (ml/min) | 86.1 (28.8) |
| Creatinine (mg/dl) | 1.01 (0.47) |
| Characteristics of the receptors (n=114) | |
| Age (years) | 56.8 (10.9) |
| Sex male n (%) | 71 (62.3) |
| Retransplant n (%) | 10 (8.8) |
| HTN n (%) | 91 (79.8) |
| Hemodialysis time (years) | 3.2 (2.4) |
| HHLA incompatibilities | 3.3 (1.04) |
| Induction treatment n (%) | |
| OKT3 | 2 (1.8) |
| Basiliximab | 20 (18.3) |
| Daclizumab | 35 (32.1) |
| Polyclonal antibodies | 39 (35.8) |
| Without antibodies | 13 (12) |
| First immunosuppressant n (%) | |
| Cyclosporine | 33 (30.2) |
| Tacrolimus | 62 (56.8) |
| Sirolimus | 6 (5.5) |
| Everolimus | 8 (7.5) |
| Second immunosuppressant n (%) | |
| Mycophenolate | 88 (80.7) |
| Azathioprine | 21 (19.3) |
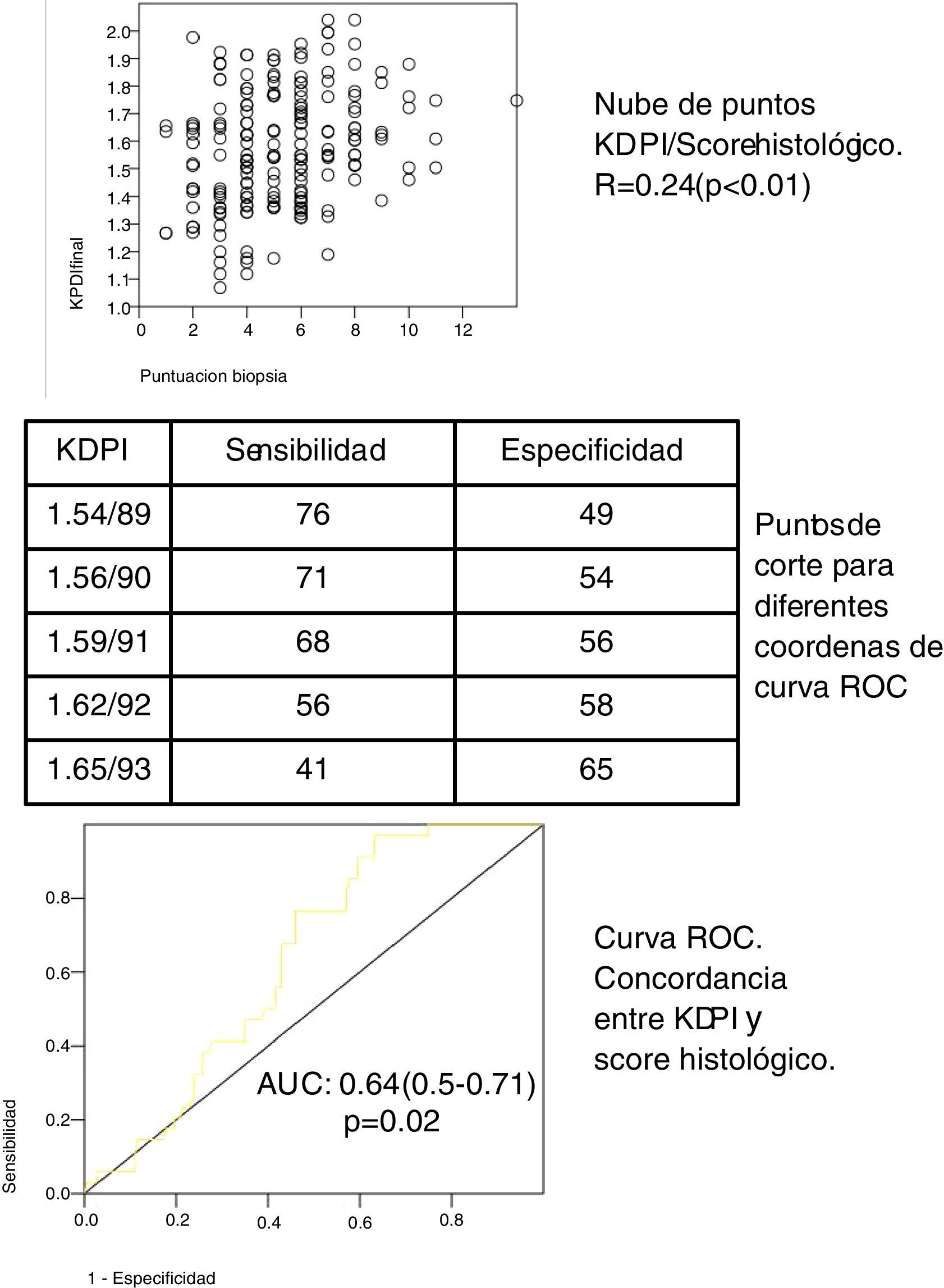
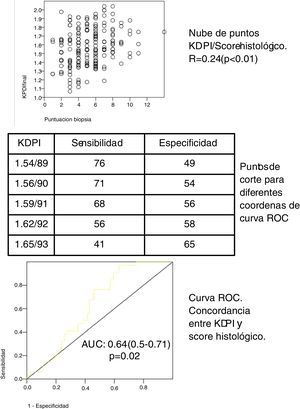
In our cohort of 197 grafts, the variable KDPI score is a continuous distribution (mean 1.58/90, SD 0.21, minimum 1.07/57, maximum 2.04/99). The median score of the biopsies was 5 (range 1–7) for the eligible grafts and 8 (range 8–14) for the non elegible grafts. The biopsy score and the KDPI score show a direct correlation (see scatter plot, Fig. 1) with Spearman correlation coefficient 0.24 (p<0.01). The mean KDPI score from elegible and non elegible grafts was significantly different (1.56/89, SD 0.22 vs 1.66/93, SD 0.15 respectively) (p<0.01). A ROC curve was constructed to identify the different KDPI scoring cut points (Fig. 1). The agreement between both scores is discrete, although significant (AUC 0.64, 95% CI: 0.59–0.71). Fig. 1 shows the sensitivity and specificity indexes for different cut points. For a cut-off value of KDPI of 1.59/91, the sensitivity is 68% and the specificity is 56%. The negative predictive value for a score of 1.59 is 65%. Thus with a score lower than 1.59, 65% of the biopsies could be elegible.
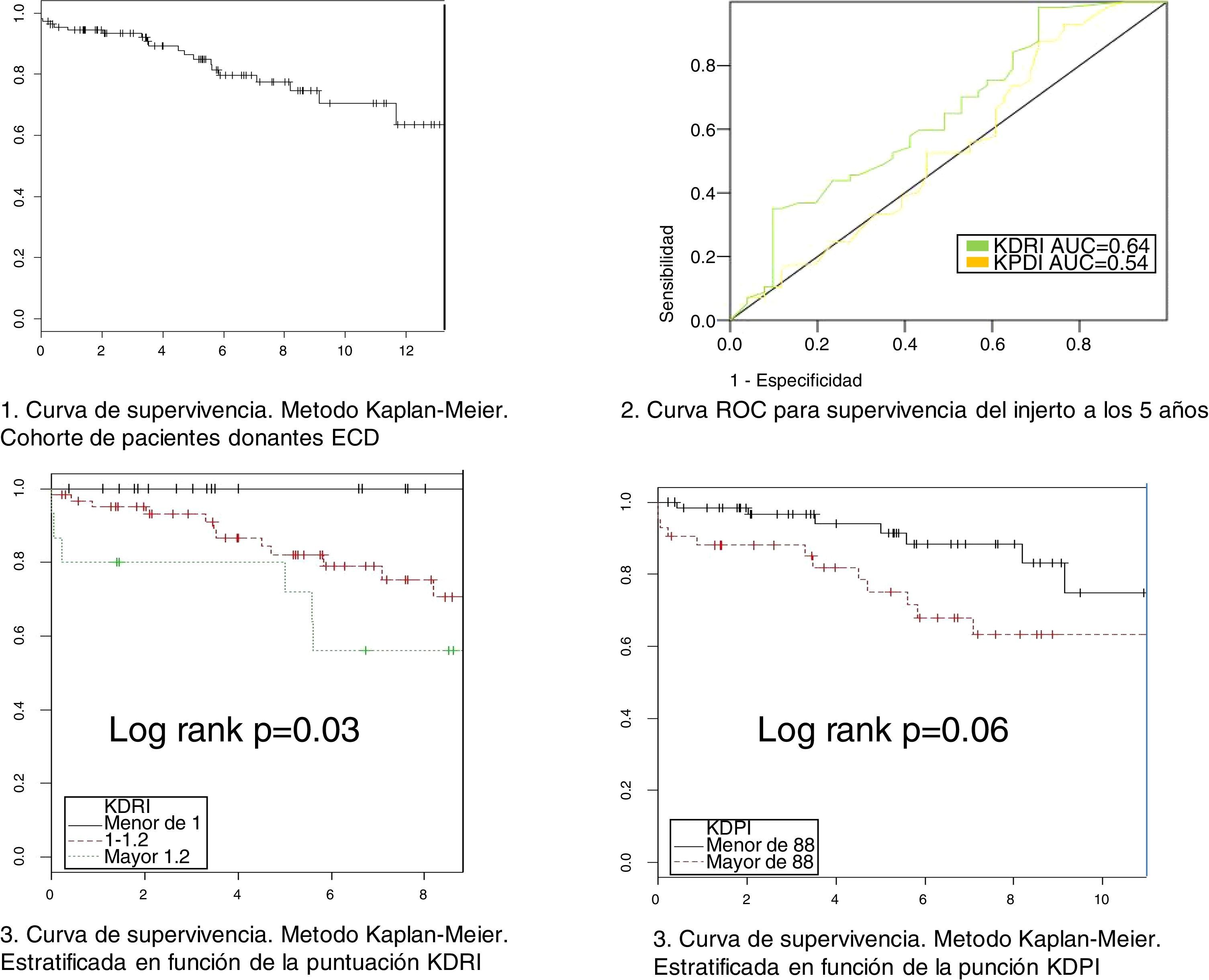
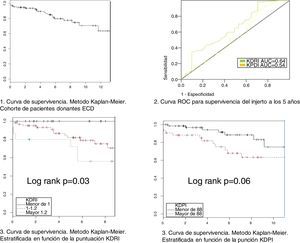
Relationship between KDPI, KDRI and histology with survivalThe variables KDPI score (mean 1.47/86, SD 0.15, min 1.08/59 and max 1.88/97) and KDRI (mean 1.08, SD 0.15, min 0.73, max 1.6) have a normal distribution. The median score of the biopsy is 4 points. The mean survival of the grafts is 5.4 years (SD 3.74) with a maximum follow-up of 13 years (see survival curve, Fig. 2). Survival at one year and at 5 years is 94.4% and 84.8% respectively. At the end of the follow-up, 74 grafts (67.9%) were still functioning, 17 patients (15.6%) were on dialysis and 18 patients (16.5%) had died.
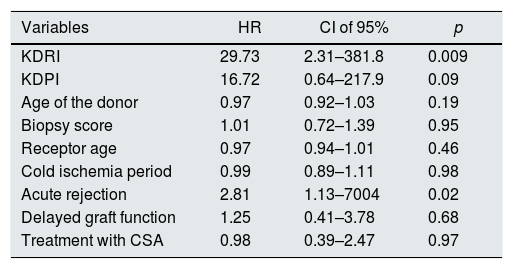
We have performed a multivariate analysis using Cox regression to identify variables related to graft survival. Initially, a bivariate analysis was performed to identify the variables showing a statistically significant influence on graft survival (Table 2). The variables with statistical significance were acute rejection, KDPI and KDRI. In the analysis adjusted for acute rejection (Table 3), the KDPI and KDRI scores were associated with graft survival. Fig. 2 shows the survival curves stratified by the KDPI and KDRI scores at different cut-off points. For a KDPI score of 88% and a KDRI score of 1.2, the survival HR was 2.45 (95% CI: 0.99–6.08, p=0.05) and 2.82 (95% CI: 1.11–7.13, p=0.02). The age of the donor was not a determinant of graft survival in our patient cohort. The predictive power for survival at 5 years was 0.54 and 0.64 for KDPI and KDRI, respectively (Fig. 2).
Univariate analysis of survival by Cox regression.
| Variables | HR | CI of 95% | p |
|---|---|---|---|
| KDRI | 29.73 | 2.31–381.8 | 0.009 |
| KDPI | 16.72 | 0.64–217.9 | 0.09 |
| Age of the donor | 0.97 | 0.92–1.03 | 0.19 |
| Biopsy score | 1.01 | 0.72–1.39 | 0.95 |
| Receptor age | 0.97 | 0.94–1.01 | 0.46 |
| Cold ischemia period | 0.99 | 0.89–1.11 | 0.98 |
| Acute rejection | 2.81 | 1.13–7004 | 0.02 |
| Delayed graft function | 1.25 | 0.41–3.78 | 0.68 |
| Treatment with CSA | 0.98 | 0.39–2.47 | 0.97 |
In our cohort of ECD patient, we found a moderate correlation between KDPI score and the preimplantation renal biopsy. Both the KDPI and KDRI scores are related to survival and both are potentially valid for donor selection in the ECD graft group.
The utility of the KDPI and the preimplantation biopsy to assess grafts from ECD donors are not established. In the present study, the KDPI score and the preimplantation renal biopsy show a moderate concordance. The mean KDPI score in this study was 1.47/86%: it is above 85%, which is the cut-off point for recommendation of biopsy in other countries10 since the percentage of discarded organs could reach 38% and exceed 60% for scores of 90 or higher.11 The percentage of discarded grafts in our cohort of patients was 10, 26 and 16% for KDPI values lower than 80, between 80 and 90, and greater than 90, respectively. Gandofini et al.11 performed a study with similar design in a large cohort of patients. The preimplantation biopsy allowed to reduce the percentage of organs that would have been discarded in KDPI scores over 80 and 90, with excellent survival results. These results make it unwise to use the KDPI in our setting as the only tool to accept or reject a kidney graft, as Pascual and Pérez state in a recent editorial in this journal12 and as other authors have proposed in other countries.13
We agree that the value of the biopsy has been overestimated and the time has come to reach a balance between clinical and histological parameters, optimizing the resources without increasing the percentage of discarded organs. The histological scores combined with KDPI have advantages over the classical SCD/ECD division, however it is necessary to establish a cut-off point to indicate the preimplantation biopsy that allows individualized decisions about the donors. We find it unlikely that a clinical trial could be conducted to assess the usefulness of the biopsy as proposed by other authors,14 given the ethical problems that could be posed by the implantation of all grafts after being aware of the biopsy results.
In our study, the KDPI and KDRI values are correlated with graft survival. This relationship has been described by others.4,5,15,16 In our cohort, a 0.1 point increase in the KDRI score increases the risk of graft failure by 9.5% per year. As far as the KDPI score, an increase in 1 point is associated with a decreased survival of 2.5% per year. The predictive power of KDRI to estimate survival at 5 years is 64%, superior to that of KDPI, and it is identical to the values published by Rao et al.4 In our study the predictive power of the KDPI score is low, although its predictive capacity could be comparable, since acute rejection is a confounding variable in the relationship KDPI and graft survival. In any case, performing the biopsy causes a delay in the transplant and allows to have all the data from the recipients, so that the calculation of the complete KDRI score (including the data of potential recipients) can be accomplished which improves the predictive value. The information from the biopsy plus the KDRI score in grafts of higher KDPI score (and supposedly of worse quality) would allow to select the ideal recipient with greater possibilities of survival for each graft. Other articles17 have shown a significant association between KDRI and 4-year follow-up graft survival in a cohort of patients with short cold ischemia; this confirm that KDRI is a useful tool for assessing the donor ECD, with independence of the period of cold ischemia. The hazard ratios (HR) found in our study are very high and have a very wide confidence interval (CI). The elevation of one point in the scores increases the risk of graft failure between 23 and 25 times. In clinical practice the graft score is usually distributed in 0.1 point changes, which means HR between 2.3 and 2.53 per 0.1 points per year. The high IC is due to the sample size and the large number of variables that are introduced in each of the scores. By dichotomizing the variables, the magnitude of the HR and the CIs is reduced, which gives validity to our model.
The presence of renal lesions in the donor's biopsy is associated with acute rejection and worse kidney function and reduced survival.18–22 Our group has demonstrated the validity of the renal biopsy to identify the presence of histopathological lesions that condition a worse outcome.23 In a recent study, the biopsy score correlated with renal function during the first year after transplantation.24 However, in the present work we have not been able to demonstrate a relationship between the biopsy score and graft survival. This result should be interpreted adequately, since the donor biopsy was used to select the suitability of the organ, eliminating grafts with high scores, which makes difficult to compare survival. By contrast, Han et al.17 have shown the relationship between biopsy score on graft survival, although their cohort of patients had not been selected by biopsy and the histological score was obtained a posterior with a score distribution very different from ours. The majority of the grafts analyzed by Han et al.17 have a score of 0, which corresponds to grafts from standard donors. A comparison of survival of score of 0 versus score greater than 0, is really a comparison between survival of ECD donors against donors without expanded criteria. In addition, the proportion of ECD patient was 11%, which is very different from the distribution of donors in Spain.
Although our study has limitations because it is retrospective and from a single center, it also has some strengths. The volume of patients is relatively high considering that it is a single-center study and the follow-up period is much longer than the existing in the literature. It focuses on the analysis a concordance between the biopsy findings and the KDPI score, which could help the different renal transplant groups to select a cut-off point of KDPI to indicate the performance of renal biopsy as a complementary tool. In addition, the being performed in a single center can be considered a strength, since both the biopsy assessment and histological processing have been performed by a homogeneous group of experts in the assessment of preimplantation biopsies, which minimizes interobserver variability. In fact, a recent study24 has shown the importance of interobserver variability, as well as the superiority of paraffin processing in this type of studies, especially for organs with higher scores in preimplant biopsy.
In conclusion, (1) the KDPI and histological scores (preimplantation biopsy) show a moderate concordance. The value of both scores combined is to be determined. It is necessary to carry out prospective studies to select an adequate KDPI cut-off point, although with the accumulated evidence it could be reasonable to set a KDPI score between 85 and 91; (2) the KDPI scores, and especially KDRI, are valid to estimate the survival of the grafts and can be used in combination with the biopsy to make decisions in the graft group from ECD.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: del Moral Martín RMG, Retamero Díaz JA, Cava Molina M, Cobacho Tornel BM, Bravo Soto J, Osuna Ortega A, et al. Validación del KDRI/KPDI para la selección de donantes renales con criterios expandidos. Nefrología. 2018;38:297–303.