Atrial fibrillation (AF) represents an important social and healthcare problem. There is wide variability in the prevalence of this arrhythmia in studies analysing patients on haemodialysis (HD).
ObjectiveTo investigate the prevalence, clinical profile and therapeutic management of patients with AF on HD in Andalusia.
MethodsWe asked the public healthcare system of Andalusia to provide us with the number of patients who were being treated with HD. We asked attending nephrologists from all hospital and outpatient centers in 5 of the 8 Andalusian provinces to perform an electrocardiogram and to fill out a questionnaire on patients selected by simple random sampling.
ResultsA total of 2348 patients were being treated with HD in the 5 provinces included in the study. The estimated sample size was 285 patients. We obtained an electrocardiogram and information from 252 patients (88.4%); mean age 65.3±16 years; 40.9% women. Sixty-three patients (25%) had AF. Of these, 36 (14.3%) had AF in the recorded ECG and in the rest it had been documented previously. In the multivariate analysis, older age (OR: 1.071; 95% CI: 1.036–1.107; p=0.000) and greater time on HD (OR: 1.009; 95% CI: 1.004–1.014; p=0.000) were independently associated with the presence of AF. Of the patients with AF, 41.3% were on anticoagulant treatment at the time of the study; and 41.2% were on antiplatelet agents.
ConclusionsAF in dialysis units is an important finding. Establishing the risk–benefit ratio of anticoagulant treatment constitutes a real challenge. Well-designed clinical trials are pivotal in order to define the rational use of antithrombotic drugs.
La fibrilación auricular (FA) es un importante problema social y sanitario. Existe una amplia variación en la prevalencia de esta arritmia en los estudios que analizan a los pacientes en hemodiálisis (HD).
ObjetivoInvestigar la prevalencia, perfil clínico y manejo terapéutico de los pacientes con FA en HD en Andalucía.
MétodosSolicitamos al sistema sanitario público de Andalucía el número de pacientes que estaban siendo tratados con HD. Pedimos a los nefrólogos responsables de todos los centros hospitalarios y extrahospitalarios de 5 de las 8 provincias de Andalucía que realizaran un electrocardiograma y cumplimentaran un cuestionario en pacientes seleccionados por un muestreo aleatorizado simple.
ResultadosEstaban en HD 2.348 pacientes en las 5 provincias incluidas. El tamaño muestral estimado fue 285 pacientes. Obtuvimos electrocardiograma e información de 252 (88,4%). Edad media 65,3 ± 16 años; 40,9% mujeres. Tenían FA 63 pacientes (25%). De estos, 36 (14,3%) tenían FA en el registro realizado y en el resto había sido documentada previamente. En el análisis multivariante, mayor edad (OR: 1,071; IC 95%: 1,036-1,107; p = 0,000) y mayor tiempo en HD (OR: 1,009; IC 95%:1,004-1,014; p = 0,000) se asociaron de forma independiente con la FA. De los pacientes con FA, el 41,3% estaban en tratamiento anticoagulante en el momento del estudio y el 41,2% con antiagregantes.
ConclusionesLa FA en las unidades de diálisis es un importante hallazgo. Establecer la relación riesgo-beneficio del tratamiento anticoagulante constituye un auténtico reto. Son necesarios ensayos clínicos bien diseñados para establecer el uso racional del tratamiento antitrombótico.
Atrial fibrillation (AF) is the most significant sustained arrhythmia from social and health care perspectives. AF is frequent and requires specific therapy to prevent complications such as those related to thromboembolism. Thus it demands an important allocation of resources involving a number of health professionals from different areas of medicine.
The relationship between end-stage renal disease (ESRD) and cardiovascular disease is well recognized1 among nephrologists, but specific concern physician about AF is relatively recent.2
During the recent years a number of studies have analyzed the prevalence and incidence of AF in dialysis patients3–7 and the prevalence varied from 4% to 27%.4
The overall beneficial effect of anticoagulant treatment for the prevention of thromboembolism observed in the general population has not been documented in dialysis patients. Therefore, there is controversy about the use of anticoagulant treatment in dialysis patients that basically relies on the physician criteria.8
The present study was performed in the south region of Spain, Andalusia; the aim of this research was to determine the prevalence of AF in this hemodialysis population, characterize the clinical profile of patients with AF and analyze the use of antithrombotic treatment in routine clinical practice.
Patients and methodsAndalusia (South of Spain) has a population of 8,400,000 inhabitants. The treatment of end stage renal disease patients (ESRD) including dialysis and transplantation is managed by the Andalusian Health Service, which has a registry of patients under treatment “Information System of the Transplant Coordination of Andalusia” (SICATA). In December 2014, SICATA provided information on the number and distribution of patients in the hemodialysis centers of the region (both, in and out of hospital centers).
The sample size was calculated taking into consideration the wide variability on the prevalence of AF. To achive estimation accuracy of 5% with a confidence interval of 95%, and an expected prevalence of up to 25% in a total population of 2348, the calculated sample size was 285 patients. Patients were distributed among the centers in proportion to the number of patients dialyzed in each center.
The nephrologists responsables of the hemodialysis units from the 5 provinces participating in the study were contacted: Almería, Córdoba, Granada, Jaén and Málaga. The nephrologists were asked for their collaboration and they were informed about the number of patients that should be included in each unit; this number was calculated based on the total number of patients being dialyzed in a given unit. Patients had to be selected at random with the only exclusion criteria of being <18 years of age or refusal to sign informed consent.
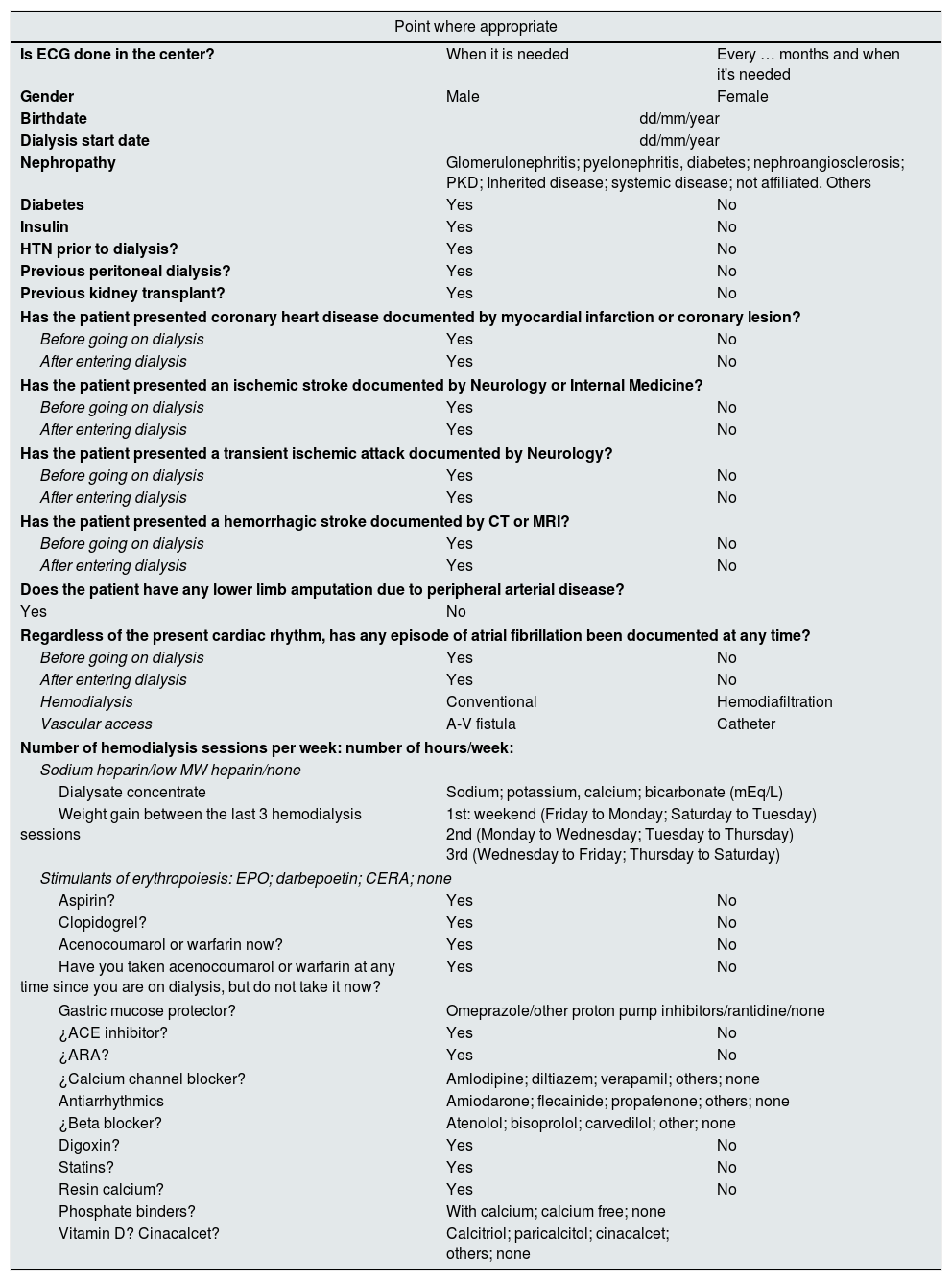
Patients had an electrocardiogram (ECG) done before starting the dialysis session or during the first hour and they were asked to complete a questionnaire that helped to define the Nephologists clinical profile and the treatment being followed. The patient was considered to have AF if at any time there had been electrocardiographic documentation of AF (Table 1).
Questionnaire to establish the clinical profile of patients, the characteristics of hemodialysis and the medical treatment.
| Point where appropriate | ||
|---|---|---|
| Is ECG done in the center? | When it is needed | Every … months and when it's needed |
| Gender | Male | Female |
| Birthdate | dd/mm/year | |
| Dialysis start date | dd/mm/year | |
| Nephropathy | Glomerulonephritis; pyelonephritis, diabetes; nephroangiosclerosis; PKD; Inherited disease; systemic disease; not affiliated. Others | |
| Diabetes | Yes | No |
| Insulin | Yes | No |
| HTN prior to dialysis? | Yes | No |
| Previous peritoneal dialysis? | Yes | No |
| Previous kidney transplant? | Yes | No |
| Has the patient presented coronary heart disease documented by myocardial infarction or coronary lesion? | ||
| Before going on dialysis | Yes | No |
| After entering dialysis | Yes | No |
| Has the patient presented an ischemic stroke documented by Neurology or Internal Medicine? | ||
| Before going on dialysis | Yes | No |
| After entering dialysis | Yes | No |
| Has the patient presented a transient ischemic attack documented by Neurology? | ||
| Before going on dialysis | Yes | No |
| After entering dialysis | Yes | No |
| Has the patient presented a hemorrhagic stroke documented by CT or MRI? | ||
| Before going on dialysis | Yes | No |
| After entering dialysis | Yes | No |
| Does the patient have any lower limb amputation due to peripheral arterial disease? | ||
| Yes | No | |
| Regardless of the present cardiac rhythm, has any episode of atrial fibrillation been documented at any time? | ||
| Before going on dialysis | Yes | No |
| After entering dialysis | Yes | No |
| Hemodialysis | Conventional | Hemodiafiltration |
| Vascular access | A-V fistula | Catheter |
| Number of hemodialysis sessions per week: number of hours/week: | ||
| Sodium heparin/low MW heparin/none | ||
| Dialysate concentrate | Sodium; potassium, calcium; bicarbonate (mEq/L) | |
| Weight gain between the last 3 hemodialysis sessions | 1st: weekend (Friday to Monday; Saturday to Tuesday) 2nd (Monday to Wednesday; Tuesday to Thursday) 3rd (Wednesday to Friday; Thursday to Saturday) | |
| Stimulants of erythropoiesis: EPO; darbepoetin; CERA; none | ||
| Aspirin? | Yes | No |
| Clopidogrel? | Yes | No |
| Acenocoumarol or warfarin now? | Yes | No |
| Have you taken acenocoumarol or warfarin at any time since you are on dialysis, but do not take it now? | Yes | No |
| Gastric mucose protector? | Omeprazole/other proton pump inhibitors/rantidine/none | |
| ¿ACE inhibitor? | Yes | No |
| ¿ARA? | Yes | No |
| ¿Calcium channel blocker? | Amlodipine; diltiazem; verapamil; others; none | |
| Antiarrhythmics | Amiodarone; flecainide; propafenone; others; none | |
| ¿Beta blocker? | Atenolol; bisoprolol; carvedilol; other; none | |
| Digoxin? | Yes | No |
| Statins? | Yes | No |
| Resin calcium? | Yes | No |
| Phosphate binders? | With calcium; calcium free; none | |
| Vitamin D? Cinacalcet? | Calcitriol; paricalcitol; cinacalcet; others; none | |
ARA: angiotensin receptor antagonists; CERA: continuous erythropoietin receptor activator; EPO: erythropoietin; HTN: arterial hypertension; ACEI: angiotensin-converting enzyme inhibition; CT: computed tomography; MRI magnetic resonance imaging.
The electrocardiographic records and the questionnaires were sent to the coordinating center (Complejo Hospitalario de Jaén) for the analysis of the ECG and unified management of the data.
The ECG was analyzed by an experienced cardiologist, who sought the opinion of a second cardiologist when he considered that it was appropriate. The analysis of the ECG included determination of rhythm, conduction disorders, abnormal Q waves, alterations of the repolarization and measurement of waves and intervals. The first patient's record were obtained in December 2014 and the last one in January 2016.
The arrhythmia was considered as permanent and non-permanent following the criteria of epidemiological records of AF in the Spanish population (OFRECE study),9 which assumes that it is permanent if the arrhythmia is present in the ECG performed on the day of the examination. The rest of AF were grouped as not permanent; these include: first episode AF, paroxysmal AF, persistent AF and persistent AF of long duration, as defined by the guide of the European Society of Cardiology, which is assumed by the Spanish Society of Cardiology,10 and also following the criteria of the OFRECE9 study.
The AF was analyzed in relation to the demographic characteristics, clinical profile, electrocardiographic findings and dialysis characteristics.
Patients were considered to have diabetes if they were on treatment for glycemic control and they were hypertensive if they were on hypotensive drugs. The diagnosis of transient ischemic attack (TIA) had to be made by a neurologist and the diagnosis of cerebrovascular accident (CVA) also required documentation by imaging techniques. The patient was considered to have coronary disease if he had an acute myocardial infarction or had significant coronary artery stenosis documented by coronary arteriography.
To compare means from patients with and without AF we used the Student t test or the nonparametric Mann–Whitney test. Qualitative variables were compared using the Chi-square test. Analysis of the association of AF with the different variables was done by multivariate logistic regression analysis; the odds ratio (OR) and 95% confidence intervals (CI) were obtained. A value of p<0.05 was considered statistically significant. The statistical analysis was done with the statistical package SPSS for Windows.
The study was approved by the Research Ethics Committee of the study coordinating center and all patients signed the consent to participate in the study.
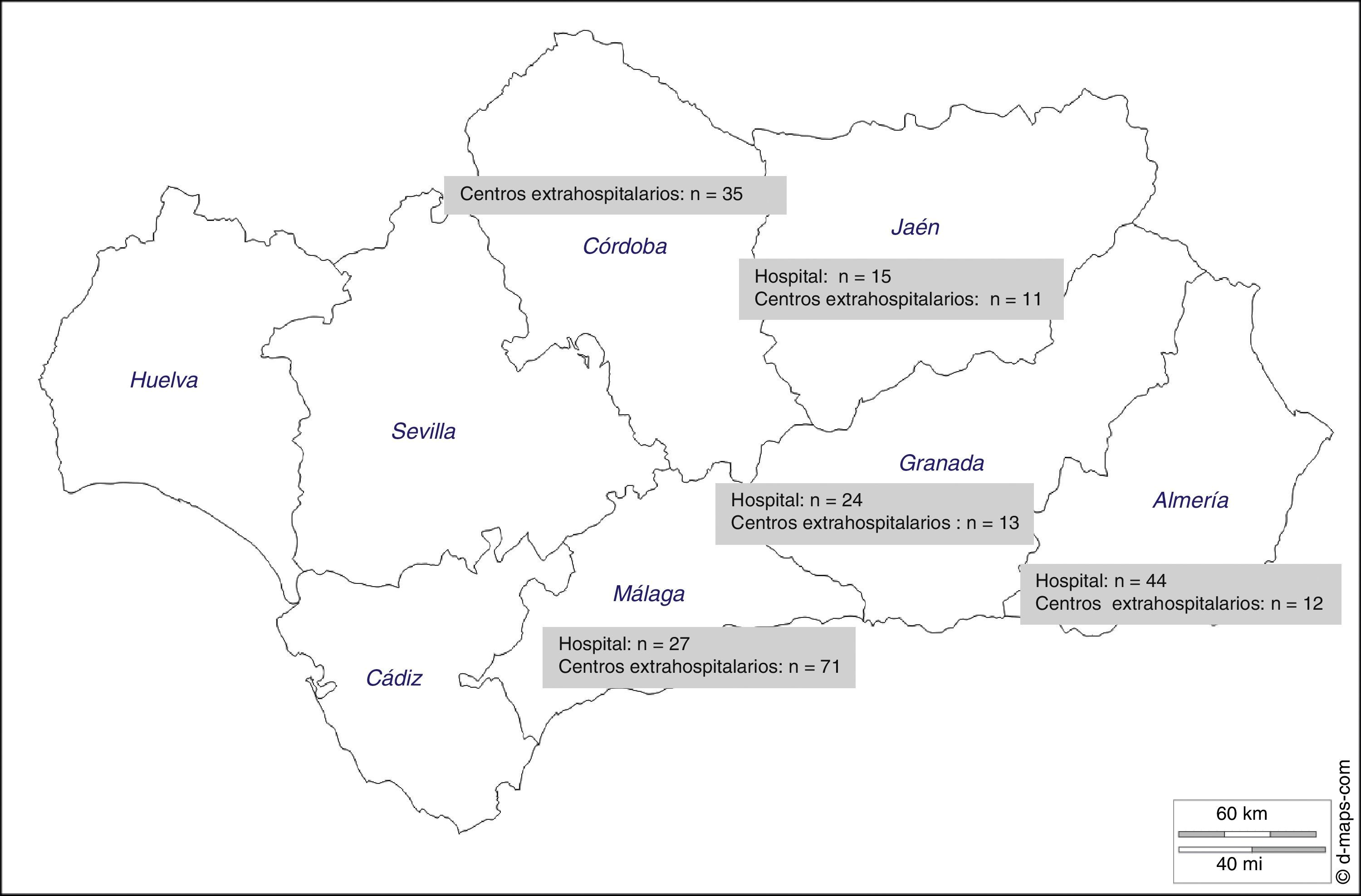
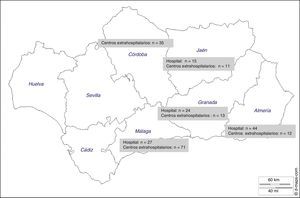
ResultsThere were 4119 patients undergoing hemodialysis in the entire region and 2348 in the 5 provinces participating in the study. The sample size calculated to estimate the prevalence of FA was n=285, however data were received from 252 patients (88.4%). Patients were dialyzed in hospital based dialysis units (n=5) and out-of-hospital dialysis units (n=19). The distribution of patients is shown in Fig. 1. The province of Málaga contributed with the greatest number of patients (n=98) and Jaén had the lowest number of patients (n=26).
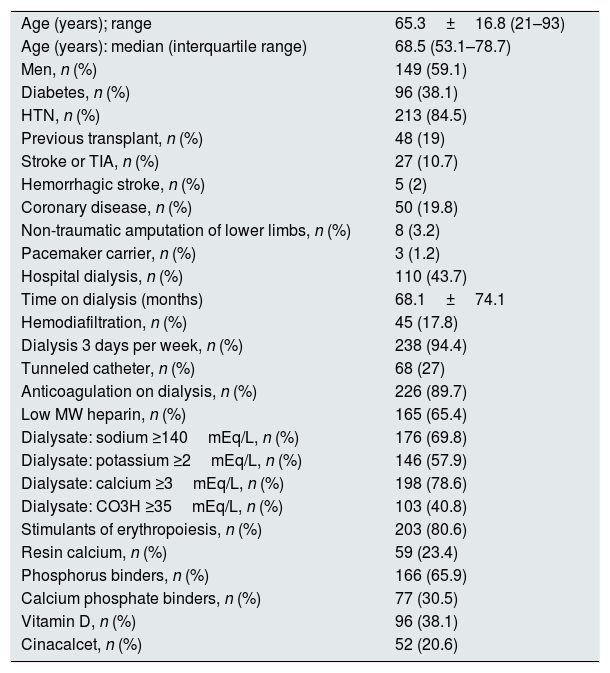
Table 2 shows the clinical profile and the characteristics of dialysis of all patients included in the study (n=252). Sixty-three patients (25%) were diagnosed with some of the clinical forms of AF. Among patients with AF, 36 (14.3% of all included) had permanent AF and 27 non-permanent AF (42.8% of all patients with FA).
Clinical profile and characteristics of dialysis of patients included in the study (n=252).
| Age (years); range | 65.3±16.8 (21–93) |
| Age (years): median (interquartile range) | 68.5 (53.1–78.7) |
| Men, n (%) | 149 (59.1) |
| Diabetes, n (%) | 96 (38.1) |
| HTN, n (%) | 213 (84.5) |
| Previous transplant, n (%) | 48 (19) |
| Stroke or TIA, n (%) | 27 (10.7) |
| Hemorrhagic stroke, n (%) | 5 (2) |
| Coronary disease, n (%) | 50 (19.8) |
| Non-traumatic amputation of lower limbs, n (%) | 8 (3.2) |
| Pacemaker carrier, n (%) | 3 (1.2) |
| Hospital dialysis, n (%) | 110 (43.7) |
| Time on dialysis (months) | 68.1±74.1 |
| Hemodiafiltration, n (%) | 45 (17.8) |
| Dialysis 3 days per week, n (%) | 238 (94.4) |
| Tunneled catheter, n (%) | 68 (27) |
| Anticoagulation on dialysis, n (%) | 226 (89.7) |
| Low MW heparin, n (%) | 165 (65.4) |
| Dialysate: sodium ≥140mEq/L, n (%) | 176 (69.8) |
| Dialysate: potassium ≥2mEq/L, n (%) | 146 (57.9) |
| Dialysate: calcium ≥3mEq/L, n (%) | 198 (78.6) |
| Dialysate: CO3H ≥35mEq/L, n (%) | 103 (40.8) |
| Stimulants of erythropoiesis, n (%) | 203 (80.6) |
| Resin calcium, n (%) | 59 (23.4) |
| Phosphorus binders, n (%) | 166 (65.9) |
| Calcium phosphate binders, n (%) | 77 (30.5) |
| Vitamin D, n (%) | 96 (38.1) |
| Cinacalcet, n (%) | 52 (20.6) |
ACV: stroke; TIA: transient ischemic attack; HTN: arterial hypertension; MW: molecular weight.
At the initiation of chronic hemodialysis 27 patients (10.7%) had been diagnosed of AF in any of their clinical forms.
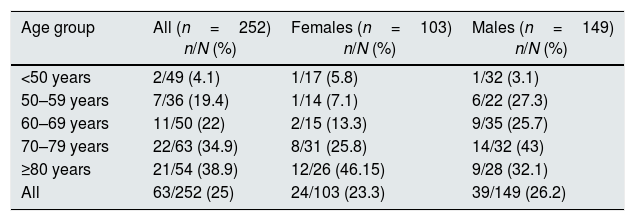
Table 3 shows the prevalence of AF in relation to the gender and the age range.
Distribution of atrial fibrillation by gender and age range.
| Age group | All (n=252) n/N (%) | Females (n=103) n/N (%) | Males (n=149) n/N (%) |
|---|---|---|---|
| <50 years | 2/49 (4.1) | 1/17 (5.8) | 1/32 (3.1) |
| 50–59 years | 7/36 (19.4) | 1/14 (7.1) | 6/22 (27.3) |
| 60–69 years | 11/50 (22) | 2/15 (13.3) | 9/35 (25.7) |
| 70–79 years | 22/63 (34.9) | 8/31 (25.8) | 14/32 (43) |
| ≥80 years | 21/54 (38.9) | 12/26 (46.15) | 9/28 (32.1) |
| All | 63/252 (25) | 24/103 (23.3) | 39/149 (26.2) |
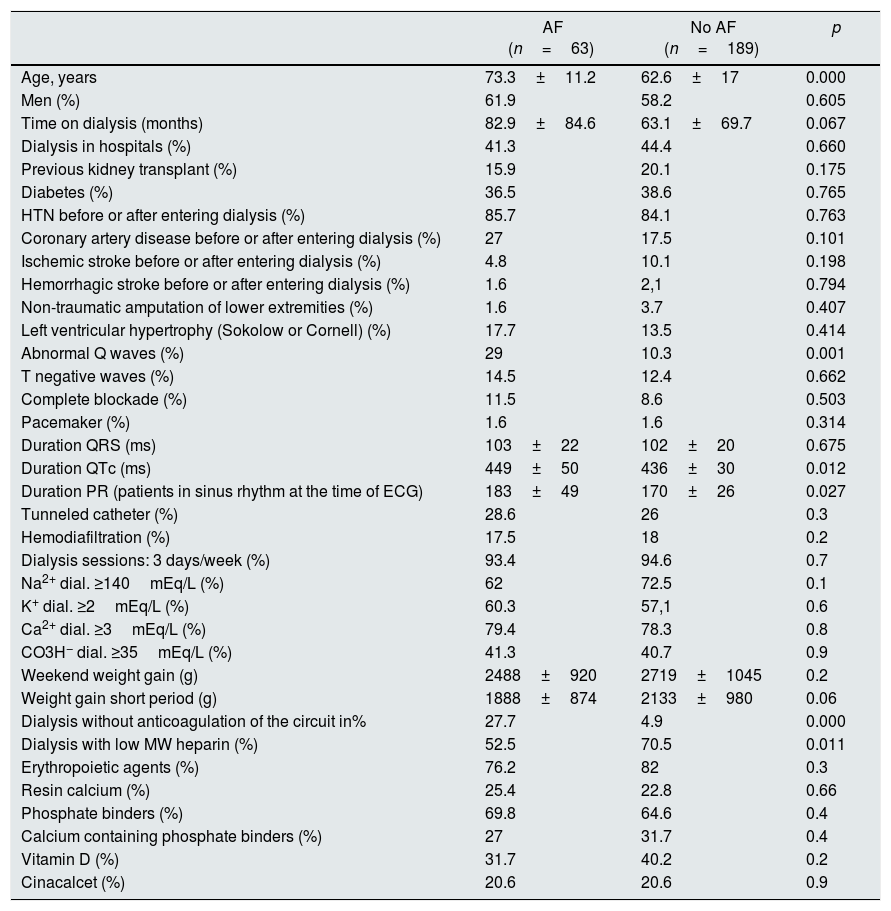
In the univariate analysis, age, abnormal Q waves and the duration of the QTc and PR intervals (PR analyzed in patients diagnosed with AF, but who were in sinus rhythm in the ECG of the day of the examination, that is, patients with non-permanent AF) showed significant differences between patients with and without AF (Table 4).
Differences in the clinical profile and characteristics of dialysis between patients with or without the diagnosis of AF.
| AF (n=63) | No AF (n=189) | p | |
|---|---|---|---|
| Age, years | 73.3±11.2 | 62.6±17 | 0.000 |
| Men (%) | 61.9 | 58.2 | 0.605 |
| Time on dialysis (months) | 82.9±84.6 | 63.1±69.7 | 0.067 |
| Dialysis in hospitals (%) | 41.3 | 44.4 | 0.660 |
| Previous kidney transplant (%) | 15.9 | 20.1 | 0.175 |
| Diabetes (%) | 36.5 | 38.6 | 0.765 |
| HTN before or after entering dialysis (%) | 85.7 | 84.1 | 0.763 |
| Coronary artery disease before or after entering dialysis (%) | 27 | 17.5 | 0.101 |
| Ischemic stroke before or after entering dialysis (%) | 4.8 | 10.1 | 0.198 |
| Hemorrhagic stroke before or after entering dialysis (%) | 1.6 | 2,1 | 0.794 |
| Non-traumatic amputation of lower extremities (%) | 1.6 | 3.7 | 0.407 |
| Left ventricular hypertrophy (Sokolow or Cornell) (%) | 17.7 | 13.5 | 0.414 |
| Abnormal Q waves (%) | 29 | 10.3 | 0.001 |
| T negative waves (%) | 14.5 | 12.4 | 0.662 |
| Complete blockade (%) | 11.5 | 8.6 | 0.503 |
| Pacemaker (%) | 1.6 | 1.6 | 0.314 |
| Duration QRS (ms) | 103±22 | 102±20 | 0.675 |
| Duration QTc (ms) | 449±50 | 436±30 | 0.012 |
| Duration PR (patients in sinus rhythm at the time of ECG) | 183±49 | 170±26 | 0.027 |
| Tunneled catheter (%) | 28.6 | 26 | 0.3 |
| Hemodiafiltration (%) | 17.5 | 18 | 0.2 |
| Dialysis sessions: 3 days/week (%) | 93.4 | 94.6 | 0.7 |
| Na2+ dial. ≥140mEq/L (%) | 62 | 72.5 | 0.1 |
| K+ dial. ≥2mEq/L (%) | 60.3 | 57,1 | 0.6 |
| Ca2+ dial. ≥3mEq/L (%) | 79.4 | 78.3 | 0.8 |
| CO3H− dial. ≥35mEq/L (%) | 41.3 | 40.7 | 0.9 |
| Weekend weight gain (g) | 2488±920 | 2719±1045 | 0.2 |
| Weight gain short period (g) | 1888±874 | 2133±980 | 0.06 |
| Dialysis without anticoagulation of the circuit in% | 27.7 | 4.9 | 0.000 |
| Dialysis with low MW heparin (%) | 52.5 | 70.5 | 0.011 |
| Erythropoietic agents (%) | 76.2 | 82 | 0.3 |
| Resin calcium (%) | 25.4 | 22.8 | 0.66 |
| Phosphate binders (%) | 69.8 | 64.6 | 0.4 |
| Calcium containing phosphate binders (%) | 27 | 31.7 | 0.4 |
| Vitamin D (%) | 31.7 | 40.2 | 0.2 |
| Cinacalcet (%) | 20.6 | 20.6 | 0.9 |
HTN: hypertension; MW: molecular weight.
Regarding characteristics of dialysis it was found that hemodialysis without heparin and the use of low molecular weight heparin as anticoagulant of the circuit were different in patients with AF. Time on dialysis and weight gain in the short interdialysis interval were at the limit of statistical significance (Table 4).
The characteristics of dialysis were also analyzed in patients who started chronic hemodialysis without the diagnosis of AF (n=225). In this group, those who presented the arrhythmia during the follow-up (n=36) did not present significant differences with respect to dialysis characteristics as compared with those who remained in sinus rhythm (n=189); the use of tunneled catheter, number of sessions and hours of dialysis per week, conventional hemodialysis or hemodiafiltration and the composition of the dialysate (sodium, potassium, calcium and bicarbonate concentration) were not different in those patients that developed AF vs. those that did not developed AF.
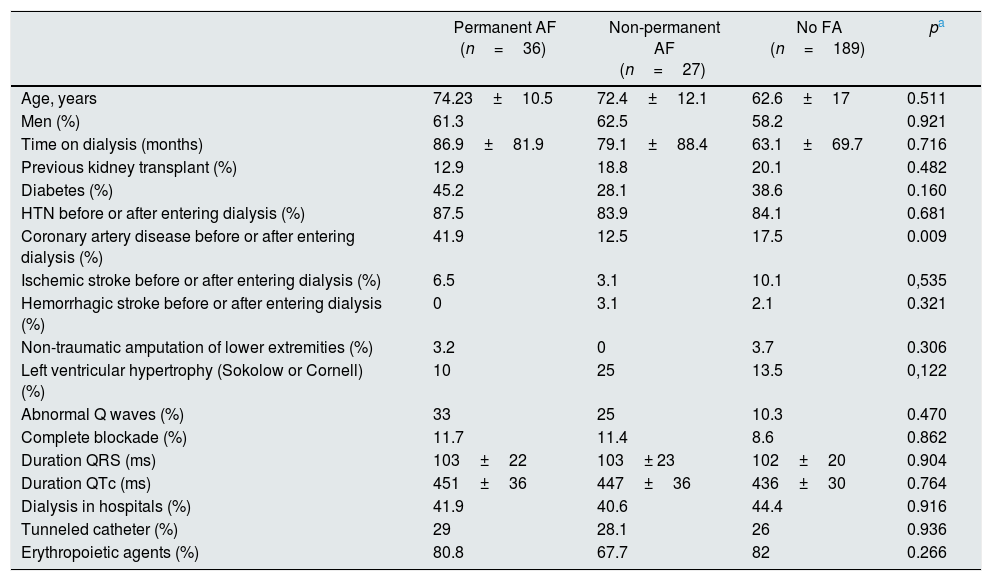
Table 5 shows the differences in the clinical profile of patients diagnosed with permanent AF and non-permanent AF.
Differences in the clinical characteristics of patients with permanent AF vs. non-permanent AF.
| Permanent AF (n=36) | Non-permanent AF (n=27) | No FA (n=189) | pa | |
|---|---|---|---|---|
| Age, years | 74.23±10.5 | 72.4±12.1 | 62.6±17 | 0.511 |
| Men (%) | 61.3 | 62.5 | 58.2 | 0.921 |
| Time on dialysis (months) | 86.9±81.9 | 79.1±88.4 | 63.1±69.7 | 0.716 |
| Previous kidney transplant (%) | 12.9 | 18.8 | 20.1 | 0.482 |
| Diabetes (%) | 45.2 | 28.1 | 38.6 | 0.160 |
| HTN before or after entering dialysis (%) | 87.5 | 83.9 | 84.1 | 0.681 |
| Coronary artery disease before or after entering dialysis (%) | 41.9 | 12.5 | 17.5 | 0.009 |
| Ischemic stroke before or after entering dialysis (%) | 6.5 | 3.1 | 10.1 | 0,535 |
| Hemorrhagic stroke before or after entering dialysis (%) | 0 | 3.1 | 2.1 | 0.321 |
| Non-traumatic amputation of lower extremities (%) | 3.2 | 0 | 3.7 | 0.306 |
| Left ventricular hypertrophy (Sokolow or Cornell) (%) | 10 | 25 | 13.5 | 0,122 |
| Abnormal Q waves (%) | 33 | 25 | 10.3 | 0.470 |
| Complete blockade (%) | 11.7 | 11.4 | 8.6 | 0.862 |
| Duration QRS (ms) | 103±22 | 103± 23 | 102±20 | 0.904 |
| Duration QTc (ms) | 451±36 | 447±36 | 436±30 | 0.764 |
| Dialysis in hospitals (%) | 41.9 | 40.6 | 44.4 | 0.916 |
| Tunneled catheter (%) | 29 | 28.1 | 26 | 0.936 |
| Erythropoietic agents (%) | 80.8 | 67.7 | 82 | 0.266 |
HTN: arterial hypertension.
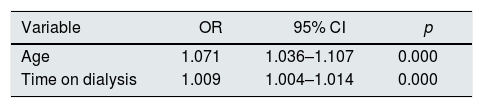
The multivariate analysis of patients who presented the arrhythmia after being included in the dialysis program shows that being older and longer time on dialysis were independently associated with the presence of AF (Table 6).
Relationship between the presence of atrial fibrillation and clinical variables, variables related to dialysis and electrocardiographic parameters.
| Variable | OR | 95% CI | p |
|---|---|---|---|
| Age | 1.071 | 1.036–1.107 | 0.000 |
| Time on dialysis | 1.009 | 1.004–1.014 | 0.000 |
Variables introduced in the analysis: age; gender; time on dialysis; dialysis in hospital; presence of diabetes, hypertension, coronary artery disease, ischemic stroke; type of vascular access; Interdialysis weight gain short period; weekend weight gain; dialysate Na≥140mEq/L; Ca≥3mEq/L; K≥2mEq/L; bicarbonate≥35mEq/L; treatment with calcium resin, vitamin D, cinacalcet, any phosphate binder and stimulants of erythropoiesis; presence of abnormal Q waves on the ECG, left ventricular hypertrophy and QTc duration.
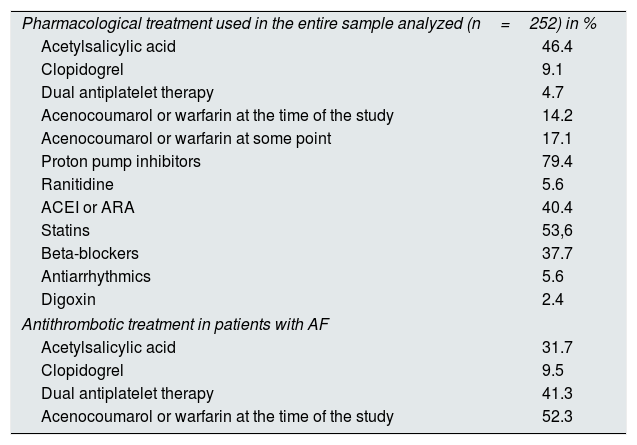
As could have been predicted, patients with AF had a high risk of thromboembolism and a high risk of bleeding, with a Congestive heart failure score, Hypertension, Age, Diabetes, Stroke, Vascular disease, Gender category (CHA2DS2VASc) of 3.5 and a Hypertension score, Abnormal Renal and Liver Function, Stroke, Bleeding, Labile INRs, Elderly, Drug or Alcohol (HAS-BLED) of 2.9. Only one patient had CHA2DS2VASc of 0 and 4 had 1. The treatment used in all patients and the antithrombotic treatment used for the prevention of thromboembolism in patients with AF is shown in Table 7.
Pharmacological treatment used in the entire sample of patients analyzed and antithrombotic treatment used for the prevention of thromboembolism in patients with atrial fibrillation.
| Pharmacological treatment used in the entire sample analyzed (n=252) in % | |
| Acetylsalicylic acid | 46.4 |
| Clopidogrel | 9.1 |
| Dual antiplatelet therapy | 4.7 |
| Acenocoumarol or warfarin at the time of the study | 14.2 |
| Acenocoumarol or warfarin at some point | 17.1 |
| Proton pump inhibitors | 79.4 |
| Ranitidine | 5.6 |
| ACEI or ARA | 40.4 |
| Statins | 53,6 |
| Beta-blockers | 37.7 |
| Antiarrhythmics | 5.6 |
| Digoxin | 2.4 |
| Antithrombotic treatment in patients with AF | |
| Acetylsalicylic acid | 31.7 |
| Clopidogrel | 9.5 |
| Dual antiplatelet therapy | 41.3 |
| Acenocoumarol or warfarin at the time of the study | 52.3 |
ARA: angiotensin receptor antagonists; AF: atrial fibrillation; ACEI: angiotensin-converting enzyme inhibitor.
The results of our study show that AF is a significant clinical problem in hemodialysis units. One out of 4 patients presented some form of AF and in 1 out of 7 the AF is permanent according to the definitions stated in the Methods section.
Patients with permanent AF had a worse cardiovascular profile, with a higher frequency of coronary disease and diabetes, although the latter did not reach statistical difference.
A high percentage of patients initiate chronic hemodialysis with AF and a high proportion of patients develop the arrhythmia during the follow up on the dialysis program.
The available information in the literature reveals a wide variation in the prevalence of this arrhythmia, ranging from 4% to 27%,4 which can be explained by differences in the age of the population analyzed, regional differences or the non uniformity in the protocols used to make the diagnosis of AF.
The information available in our country comes from studies conducted in our center that show a prevalence and incidence in the prevalent population of 13.6% and 3.1/100 patients/year of follow-up, respectively11,12 and a prevalence and incidence in the population that initiates dialysis of 12.1% and of 5.9/100 patients/year of follow-up, respectively.13 The difference observed in the prevalence of the arrhythmia in the present study as compared with our previous study from our center in the prevalent patients11 is attributable to the fact that it was carried out at the end of the nineties, when the mean age of hemodialysis patients was 5 years younger than in the present analysis and not all the clinical patterns of the arrhythmia were included.
In a similar study recently conducted in Austrian population, 2 years younger, the prevalence of the arrhythmia was 26.5%, slightly higher than the observed in the present study.14
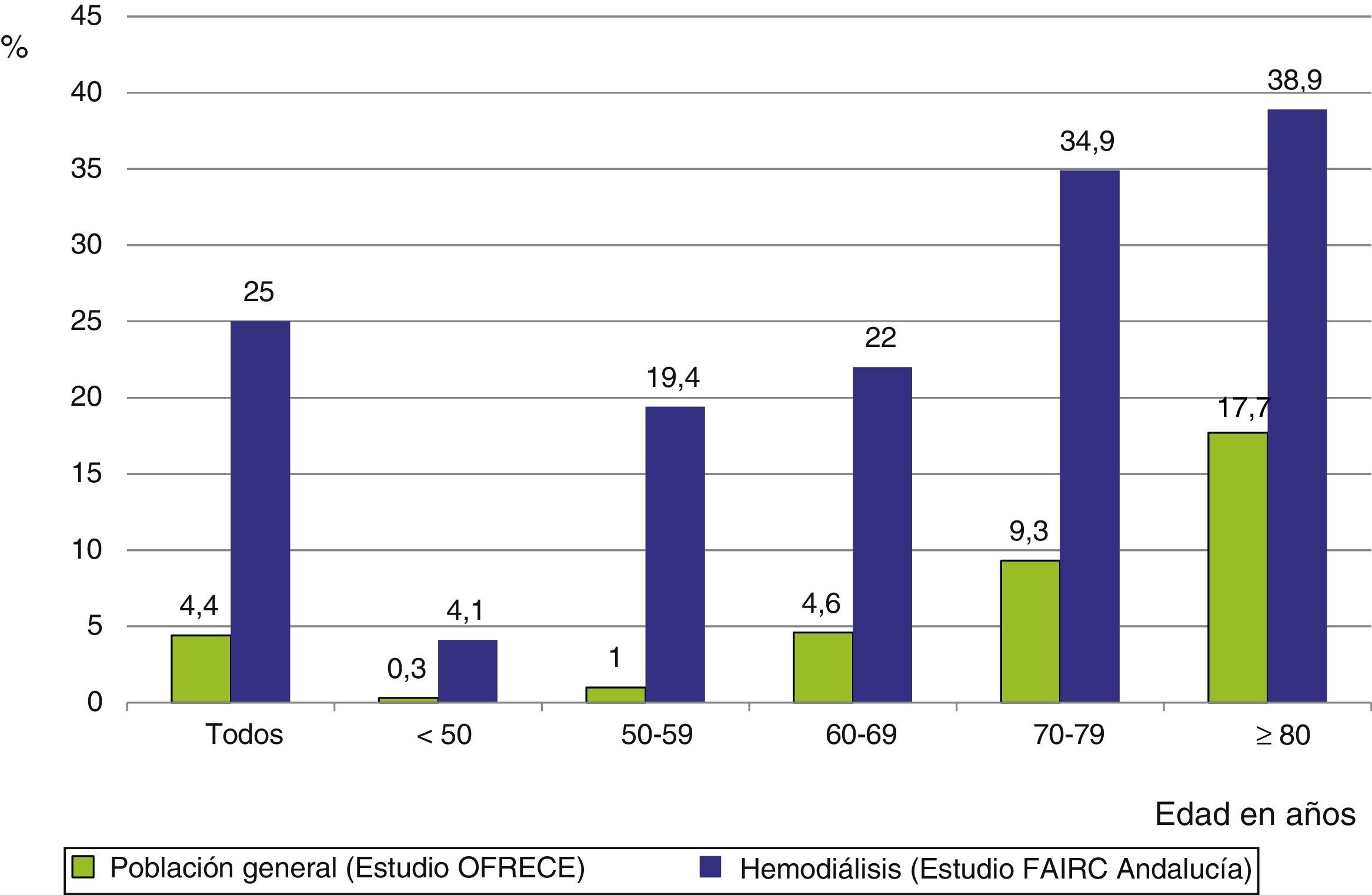
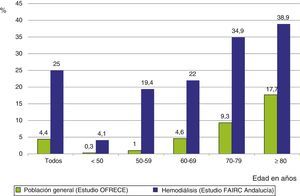
The frequency of AF in the dialysis population is 6 times greater than in the general Spanish population aged 40 or older,9 with very significant differences in the age range from 50 to 70 years, differences that were attenuated in the eighth decade of life (Fig. 2).
The relationship between older age and higher prevalence of AF shown in our study is a constant finding in all other studies, both in the general9 and dialysis population.
Although AF and ischemic heart disease share common risk factors, we have not found an association between coronary heart disease and the arrhythmia. However, in the univariate analysis there was an association of AF and the presence of abnormal Q waves, a finding that we have not observed in previous studies. One possible interpretation could be that the areas of necrosis or fibrosis in the ventricle that is represent as abnormal Q waves may be equally present in the atrial myocardium and constitute the anatomical substrate that favors the development of arrhythmia.
The intermittency of hemodialysis, with the abrupt changes of the internal environment including changes in volemia and ions, may favor the presentation of arrhythmias. The present study did not show any relationship between the presence AF and the characteristics of dialysis.
In the group of patients who were diagnosed of AF after entering the dialysis program, there was no association of the arrhythmia with the dialysis technique. As in our work, in the ESHOL study, the presence of arrhythmias in hospital admissions or deaths due arrhythmias was similar in patients on online hemodiafiltration and on standard hemodialysis, despite the fact that episodes of hypotension were less frequent with the convective technique.15
The electrolyte composition of the dialysate was not different in patients with AF. Although there is not information available from controlled studies on a causal association of electrolyte disorders and chronic AF in the dialysis population, some studies have found electrocardiographic changes, such as an long QT interval in patients with low serum levels of potassium and calcium predialysis.16 The relationship between pre-dialysis serum concentration of ions and dialysate electrolyte composition is not completely clear. A recent publication from the DOPPS study shows that predialysis hyperkalaemia is associated with the presentation of arrhythmias, but does not show an association between arrhythmias and different concentrations of potassium in the dialysate at a given level of serum potassium concentration.17
In one Spanish study, with a design different than the present study, for the same concentration of potassium in the dialysate (K: 1.5mEq/L), changes in intradialysis serum potassium in patients who presented arrhythmias were similar to those that did not experienced arrhythmias,7 which indicates that there are other factors involved, possibly alterations of the cardiac structure.
In patients in AF there is a high risk of thromboembolism as determined by the CHA2DS2VASc,18 this is the case in most studies that have analyzed this issue. In our study the risk of thromboembolism may even be underestimated, since we have not included as a variable the presence of heart failure, since there was no echocardiogram available to document ventricular function and we consider that the clinical diagnosis of heart failure in the dialysis population is difficult in a study of these characteristics.19 In the studies conducted in our institution, patients with AF present a high rate of thromboembolic events and mortality as compared to patients in sinus rhythm.13,20,21 The risk of bleeding is also high as determined by the HASBLED scale and this is concordant with the available information.22
The decision to prescribe anticoagulant treatment in these group of patients is one of the most controversial issues in the recent literature.23–25 Our study shows that 17.1% of all hemodialysis patients in or region and more than half of the patients with AF were or had been on treatment with coumarin derivatives. We believe that this is relevant information in regard to the attitude of cardiologists and nephrologists responsible for the care of these patients that face the problem of prevention of thromboembolism in dialysis patients with AF.
In a cross-sectional study conducted in 89 hemodialysis centers in Spain that represented 29% of the centers nationwide, the percentage of patients on oral anticoagulant treatment was 18.4%, very similar to the observed in the present analysis.26
Another aspect of equal importance with respect to antithrombotic treatment is the use of antiplatelet agents in dialysis patients. Considering that patients with chronic kidney disease have a high risk for atherosclerotic events, the use of acetylsalicylic acid is recommendations as primary prevention.27 However the lack of evidence of such a beneficial effect, together with the hemorrhagic risk that it entails,28,29 should make us reconsider the use of these drugs in primary prevention. In the present study, the percentage of patients on one or two antiaggregants was 51.1%, a percentage very similar to that provided by the Spanish study on anticoagulation in hemodialysis patients previously mentioned.26 A 23.8% of patients with AF did not have documented vascular disease and were receiving antiplatelet therapy. A 62% of the patients in our study that were on antiplatelet therapy did not have documented vascular disease. We assume that in the first case the treatment had been indicated for the prevention of thromboembolism and in the second as primary prevention. We believe that in both situations the indication for antiplatelet therapy should be reconsidered, as it is not indicated for the prevention of thromboembolism in patients with AF,10 and there is no evidence of a beneficial effect and still there is a considerable risk of hemorrhage.28,29
Limitations of the studyThe sample size of the study was calculated to achieve a 5% accuracy in the estimation of a proportion, with 95% interval of confidence bilateral, taking into account a 10% of losses. Finally, losses were 11.6%, so the accuracy in the present study is 5.04%.
The actual prevalence of the arrhythmia may have been underestimated. Patients without AF episodes previously documented and who were in sinus rhythm on the ECG performed on the day of examination could have presented undetected episodes at some point. This is a limitation present in all the epidemiological studies analyzing FA.9 The ECG was performed before the dialysis procedure or during the first hour, when the electrolyte changes and the ultrafiltration rate are less pronounced. In the previously mentioned Spanish study, the presentation of supraventricular arrhythmias increased during the dialysis session.7
We conclude that AF is a relevant problem in dialysis units.
The lack of management protocols, fundamentally with regard to the prevention of thromboembolism, makes therapeutic decisions a real dilemma for nephrologists and cardiologists. Controlled and randomized studies are desirable and, in the absence of these studies, the elaboration of consensus documents by experts becomes increasingly necessary.
Conflicts of interestThe authors declare no conflicts of interest.
The work was supported by the research project PI11/02275, integrated in the State Plan of I+D+i 2008-2011 of the ISCIII – “Subdirección General de Evaluación y Fomento de la Investigación” and co-sponsored by the European Fund for Regional Development (FEDER).
| Province | Center | Investigator |
|---|---|---|
| Almería | Complejo Hospitalario Torrecárdenas | Remedios Garófano López |
| Hospital de Poniente. El Ejido | Sergio García Marcos | |
| Centro de Diálisis FMC Los Arcos | Dolores Sánchez Torres | |
| Centro de Diálisis Huércal-Overa | Juan Francisco Requena Soriano | |
| Córdoba | Centro de Diálisis FMC Cabra | Fco. Javier Ariza Fuentes Soledad Gallardo Valle |
| Centro de Diálisis FMC Pintor | Fco. Javier Ariza Fuentes M. Jesús Moreno Rubio | |
| Centro de Diálisis FMC San Rafael | Isabel Berdud Godoy | |
| Centro de Diálisis Perpetuo Socorro | M. Antonia Álvarez de Lara Mateo Alcántara Crespo | |
| Centro de Diálisis Ntra. Sra. de Belén. Palma del Río | Victoria Eugenia García Montemayor | |
| Granada | Hospital Universitario Virgen de las Nieves | Pilar Galindo Sacristán |
| Carmen de Gracia Guindo | ||
| Centro de Diálisis Nevada | M. José Torres Sánchez | |
| Centro de Diálisis Guadix | M. Carmen Ruiz Fuentes | |
| Jaén | Complejo Hospitalario de Jaén | M. José García Cortés |
| Centro de Diálisis Santa Catalina. Diaverum | M. del Mar Biechy Baldán | |
| Centro de Diálisis Nefrolinares. Linares | Miguel Ángel García Pérez | |
| Málaga | Hospital Regional Universitario | Teresa Vázquez Sánchez Lara Perea Ortega |
| Centro de Diálisis Ayala. Diaverum | Tamara Jiménez Salcedo | |
| Centro de Diálisis Torremolinos. Diaverum | Luis Cermeño Marví | |
| Centro de Diálisis de la Axarquía. Diaverum | Antonio Romero Alcántara | |
| Centro de Diálisis FMC Ciudad Jardín | Seema Sujan | |
| Centro de Diálisis FMC El Cónsul | M. Victoria Moreno Muñoz Jesús Ruiz Alaminos | |
| Centro de Diálisis Braun | Elena Vaquero Párrizas Lourdes Blanca Martos Edisson Rudas Bermúdez | |
| Centro de Diálisis Hospital Quirón. Marbella | Néstor Oliva Dámaso |
A complete list of members of the research group is cited in Appendix A.
Please cite this article as: Sánchez Perales C, Vázquez Sánchez T, Salas Bravo D, Ortega Anguiano S, Vázquez Ruiz de Castroviejo E. Fibrilación auricular en los pacientes en hemodiálisis en Andalucía. Prevalencia, perfil clínico y manejo terapéutico. Nefrología. 2018;38:286–296.