La persistencia de la proteinuria nefrótica favorece la progresión hacia la insuficiencia renal. Hemos diseñado un protocolo terapéutico con sirolimus para ese grupo de pacientes implementando un estudio clínico prospectivo, intervencionista y no aleatorizado, sobre una cohorte de 13 pacientes con una edad media de 10 años; todos con síndrome nefrótico córtico-resistente primario y glomerulosclerosis focal y segmentaria; resistentes también a la ciclofosfamida, a los inhibidores de la calcineurina y al empleo de enalapril y losartán. La dosis media empleada de sirolimus fue de 3,6 mg/m2/día y el tratamiento duró 12 meses. Evaluamos la eficacia terapéutica acorde a la reducción de la proteinuria (respuesta total, parcial y ausente), y también fueron evaluadas la severidad del daño histológico pretratamiento y el tiempo previo transcurrido hasta recibir el sirolimus. Nueve de los trece pacientes tuvieron remisión parcial o total del síndrome nefrótico, y el tiempo medio previo transcurrido y la severidad del daño histológico influyeron en el tipo de respuesta. Consideramos que el sirolimus es una opción válida de tratamiento para los pacientes con síndrome nefrótico córtico-resistente, aunque probablemente sea necesario un inicio terapéutico más precoz.
Persistent nephrotic syndrome that does not respond to treatment may cause progression to kidney failure. We designed a therapeutic protocol with sirolimus for this group of patients. We conducted a prospective, interventional, time series, cohort study lasting 20 months. Thirteen patients were enrolled, with a mean age of 10 years (range: 8-18 years old) with steroid-resistant primary nephrotic syndrome and a histological diagnosis of focal and segmental glomerulosclerosis. We administered sirolimus 3.6mg/m2/day. The duration of this regimen was 12 months in responsive patients. The protocol’s efficacy was assessed according to reduction of proteinuria (3 response levels: total, partial, or no response). Severity of histological renal damage and mean time from clinical diagnosis to protocol initiation were also assessed. Nine of 13 patients responded to the treatment with sirolimus, and mean progression time and the severity of histological renal damage influenced response to therapy. We believe that sirolimus is a valid treatment option in patients with steroid-resistant nephrotic syndrome, even though this regimen probably requires an earlier treatment.
INTRODUCTION
Steroid-resistant nephrotic syndrome (SRNS) can lead to chronic renal failure.1,2 Proteinuria values in the nephrotic range provide a good marker for renal damage, and reducing these values is correlated with preserved glomerular filtration rate.3,4
The massive flow of proteins to the mesangium causes cell proliferation and the activation of chemotactic factors, with a consequent progression towards extrinsic compression of the glomerular vessels, finally leading to global and diffuse glomerulosclerosis.5,6 In order to avoid this progression, several different therapeutic options have been employed (cyclophosphamide [CPM], sodium mycophenolate, cyclosporine [CsA], levamisole, tacrolimus, angiotensin-converting enzyme [ACE] inhibitors, angiotensin receptor blockers [ARB], etc.), in some cases with fairly unsatisfactory results and severe toxic effects.7-11
We designed a treatment protocol for sirolimus (mTOR inhibitor with antiproliferative effects) for primary nephrotic patients with focal segmental glomerulosclerosis (FSGS) that are unresponsive to normal treatment.
OBJECTIVES
The primary objective was to evaluate the reduction of proteinuria in patients with primary SRNS treated with sirolimus.
Secondary objectives included: 1) evaluate the correlation between response to treatment and time elapsed from the clinical diagnosis of nephrotic syndrome to treatment with sirolimus; 2) evaluate the relationship between histological damage at the start of treatment and the type of response to treatment.
MATERIAL AND METHOD
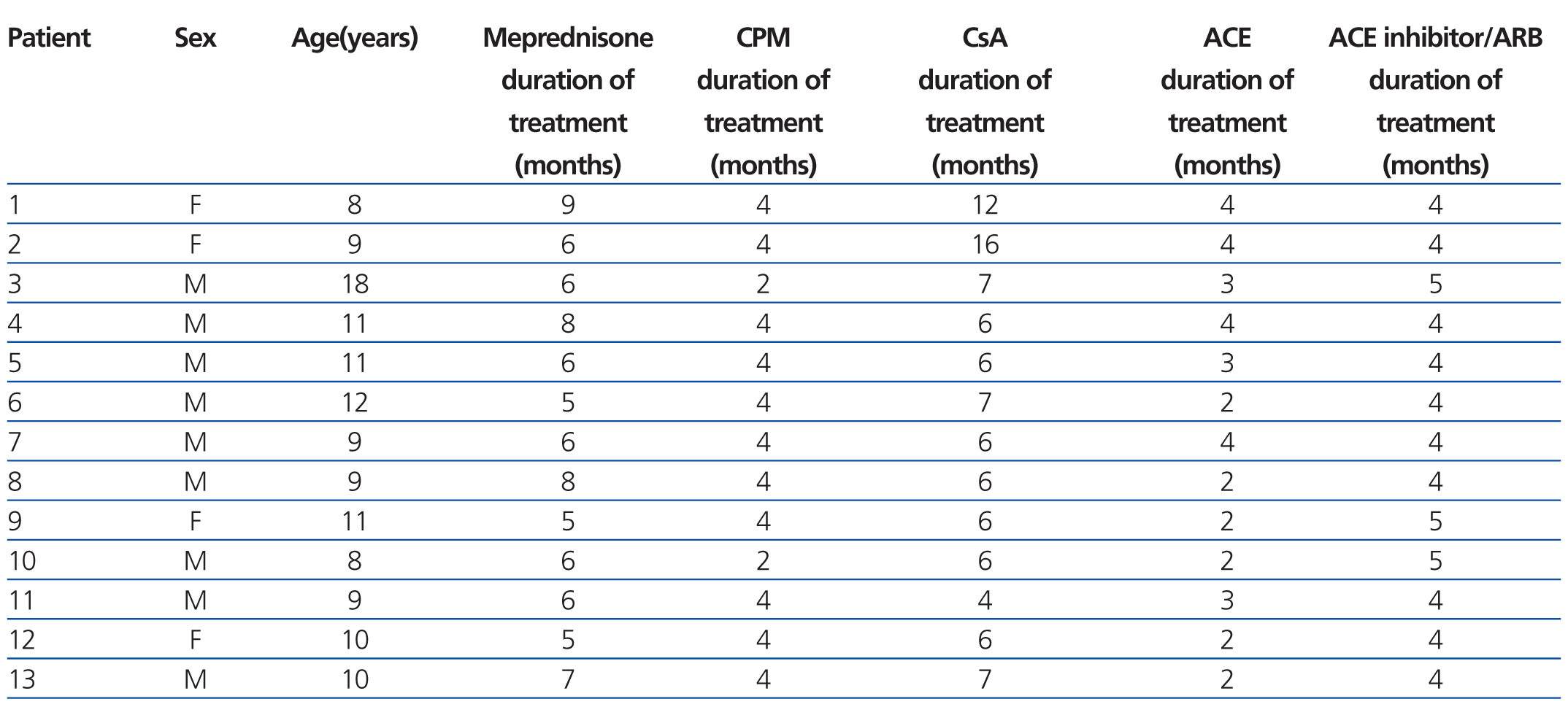
We performed a prospective, interventional, non-randomised intra-subject study, using a cohort series of 13 patients with a mean age of 10 years (range: 8-18 years) who had primary SRNS resistant to calcineurin inhibitors (CsA, tacrolimus), and cyclophosphamide. They did not respond to enalapril (EN) or losartan (LO) treatment either.
We defined nephrotic syndrome as proteinuria >40mg/m2/hour, hypoalbuminaemia <2.5g%, and frequent hypercholesterolemia above the 95th percentile for the patient’s sex and age group.12 We defined SRNS as persistent nephrotic syndrome after having completed 4 weeks of treatment with meprednisone at 48mg/m2/day (steroid dose equivalent to 60mg/m2/day of prednisone), followed by 3 consecutive pulses of 16-beta-methylprednisolone at 15mg/kg/dose.13,14
Patients were biopsied before starting treatment with CsA, and the pathological diagnosis of FSGS was defined as at least one glomerulus with a segmental lesion, which in turn was defined as the existence of at least one lobule with capillary scarring in the glomerulus examined. The diagnosis was considered to be primary in the absence of immunopathological or ultrastructural evidence of other coexisting glomerular diseases, or in the absence of a systemic disease associated with FSGS. It was not possible to perform genetic analyses of any of the patients studied.
Inclusion criteria were: nephrotic syndrome with primary FSGS, negative pregnancy test, and approved medical consent. The exclusion criteria were: creatinine clearance (CCr) <60ml/min/1.73m2, gastric or duodenal ulcers, active tumours and/or infections with or without specific treatment, diabetes, morbid obesity, vesicoureteral reflux, single kidney, or intravenous drug abuse. Finally, the criteria for interrupting treatment were a reduction in CCr>30% from initial levels for a period >3 months, leukopenia (leukocyte count <3000/mm3), refractory anaemia, active infection, persistent gastrointestinal intolerance, or lack of response in proteinuria reduction, <50% from initial values, after 6 months of treatment.
The variables evaluated included: nephrotic proteinuria, severity of the initial histological lesion, adverse events related to treatment with sirolimus, and time elapsed between establishing the clinical diagnosis and starting treatment with sirolimus.
In order to evaluate the efficacy of treatment, we established three different levels of therapeutic response: 1) total response: reduction in proteinuria <4mg/m2/hour; partial response: reduction in proteinuria of 4-40mg/m2/hour; and no response: persistent nephrotic proteinuria >40mg/m2/hour.
All histological lesions were classified by the same pathologist, who established the severity of histological renal damage according to medical literature values15 and personal experience, establishing a scale of 0-3 (absent, mild, moderate, and severe) for glomerular sclerosis, interstitial fibrosis, and vascular atrophy, respectively. Based on the level of sensitivity and specificity for each histological variable measured, a score >6 was considered to be indicative of high risk. We also evaluated the time elapsed between the clinical diagnosis of nephrotic syndrome and the start of treatment with sirolimus.
The 13 patients were receiving EN at the start of the study, with a dosage range of 0.1-0.3mg/kg/day, and LO at a dose of 0.8-1.5mg/kg/day. Sirolimus was administered at 1-5mg/m2/day (maximum dose of 5mg/day) once a day, maintaining a whole blood concentration of 7-10ng/ml.
We took monthly blood samples and tested for: creatinine (calculating CCr using the Schwartz method) uraemia, complete haemogram, cholesterolemia, triglyceridemia, LDL cholesterol (low-density lipoprotein), HDL cholesterol (high-density lipoprotein), protein electrophoresis, serum amylase, blood uric acid, serum lipase, hepatogram, serum electrolytes, and sirolimus levels in whole blood samples. We also measured urine electrolytes, proteinuria/day, and urine urea in urine samples.
If adverse events occurred related to the treatment provided, the following measures were taken:
-25% reduction of the initial sirolimus dose
-Treatment of symptoms,
-Return to initial drug dose once symptoms disappeared.
The statistical methods used for analysing the data were:
-Wilcoxon test for evaluating the probability of response based on the type of therapy employed and variation in proteinuria,
-Multiple regression analysis to evaluate the relationship between each variable evaluated (tubular atrophy, interstitial fibrosis, glomerular sclerosis, time elapsed between the clinical diagnosis of nephrotic syndrome and the start of therapy, and proteinuria prior to treatment) and variation in proteinuria during the study.
A P-value <0.05 was considered to be statistically significant. Results were expressed as mean ± SD. We used SPSS statistical software, version 13.0, for all analyses.
We obtained informed consent from all patients prior to inclusion in the study, and all steps of the treatment protocol were evaluated and approved by the ethics and research committee at our hospital. There were no conflicts of interest.
Treatment lasted a total of 12 months, and the last check-up was performed 26 months after treatment had commenced.
RESULTS
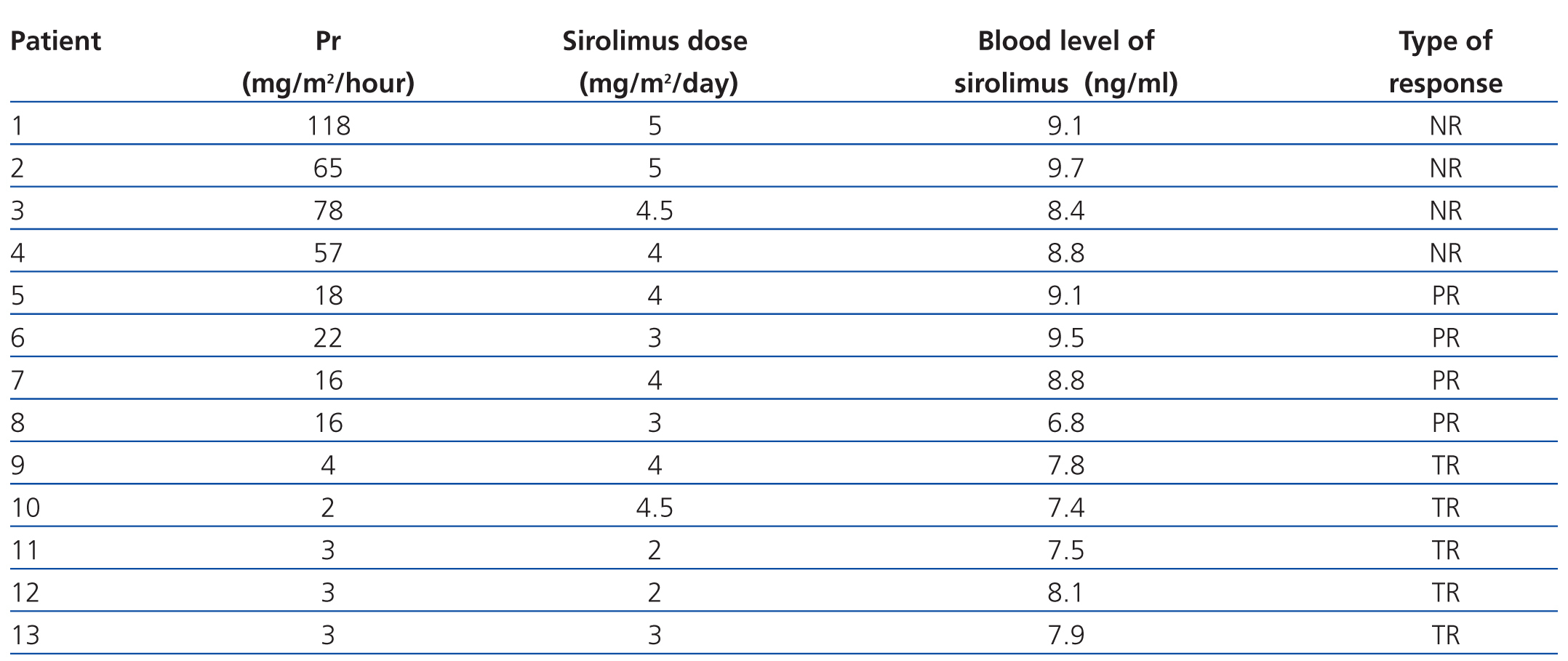
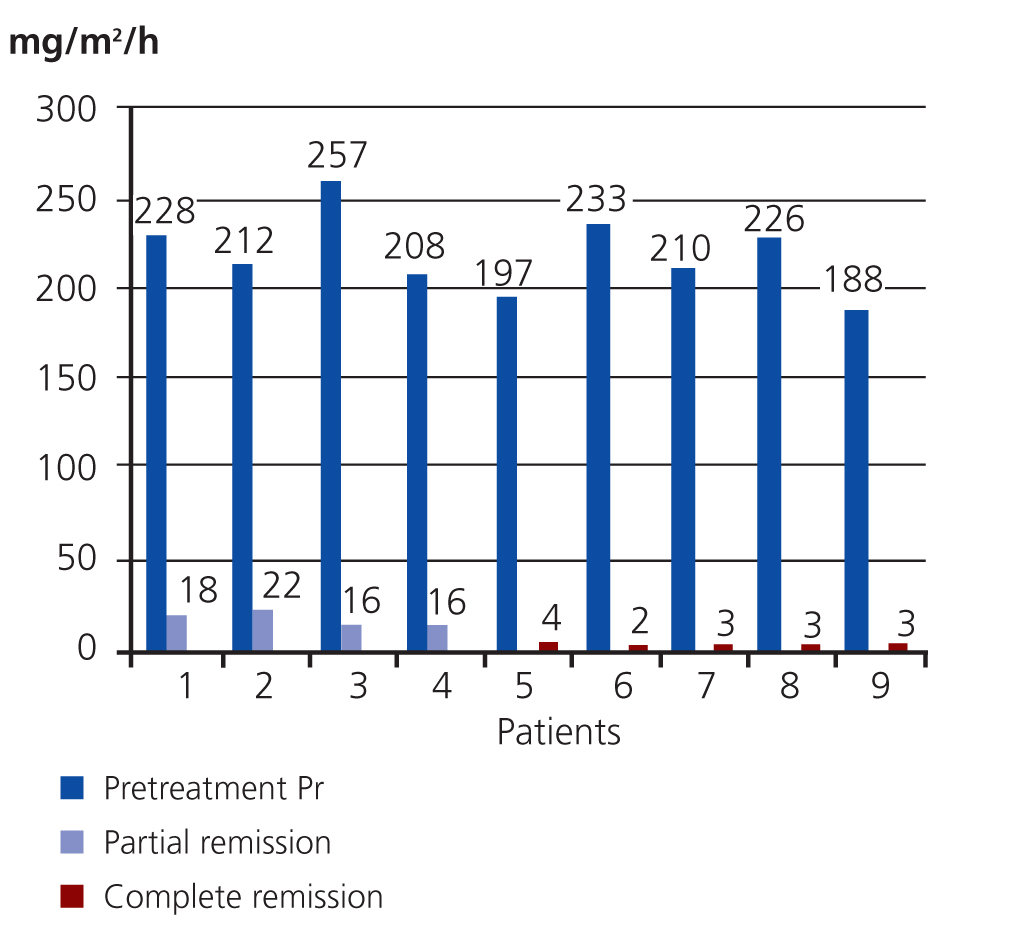
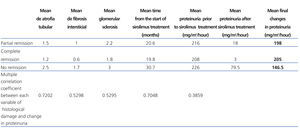
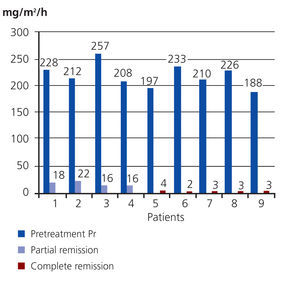
Upon completing the study, 9/13 patients had responded to treatment; of them, 5 had complete remission of disease and 4 had a partial response. The Wilcoxon test showed very significant results (P=.0002). The mean pre-treatment value for proteinuria in the 9 patients that responded was 212mg/m2/hour (SD: 20), and the mean post-treatment value in the 4 patients with a partial response was 18mg/m2/hour (SD: 3), with a 92% reduction in proteinuria. The 5 patients with a complete response had a mean post-treatment proteinuria value of 3mg/m2/hour (SD: 1), a 99% reduction in proteinuria (Figure 1).
The mean pre-treatment proteinuria value in the 4 unresponsive patients was 226mg/m2/hour (SD: 22) and the post-treatment value was 79mg/m2/hour (SD: 27). This corresponded to a 65% reduction.
During the 12 months of treatment, 2 of the patients with a partial response suffered recurrences. Both reached a partial response again after receiving traditional steroid treatment, with no changes at 1 year.
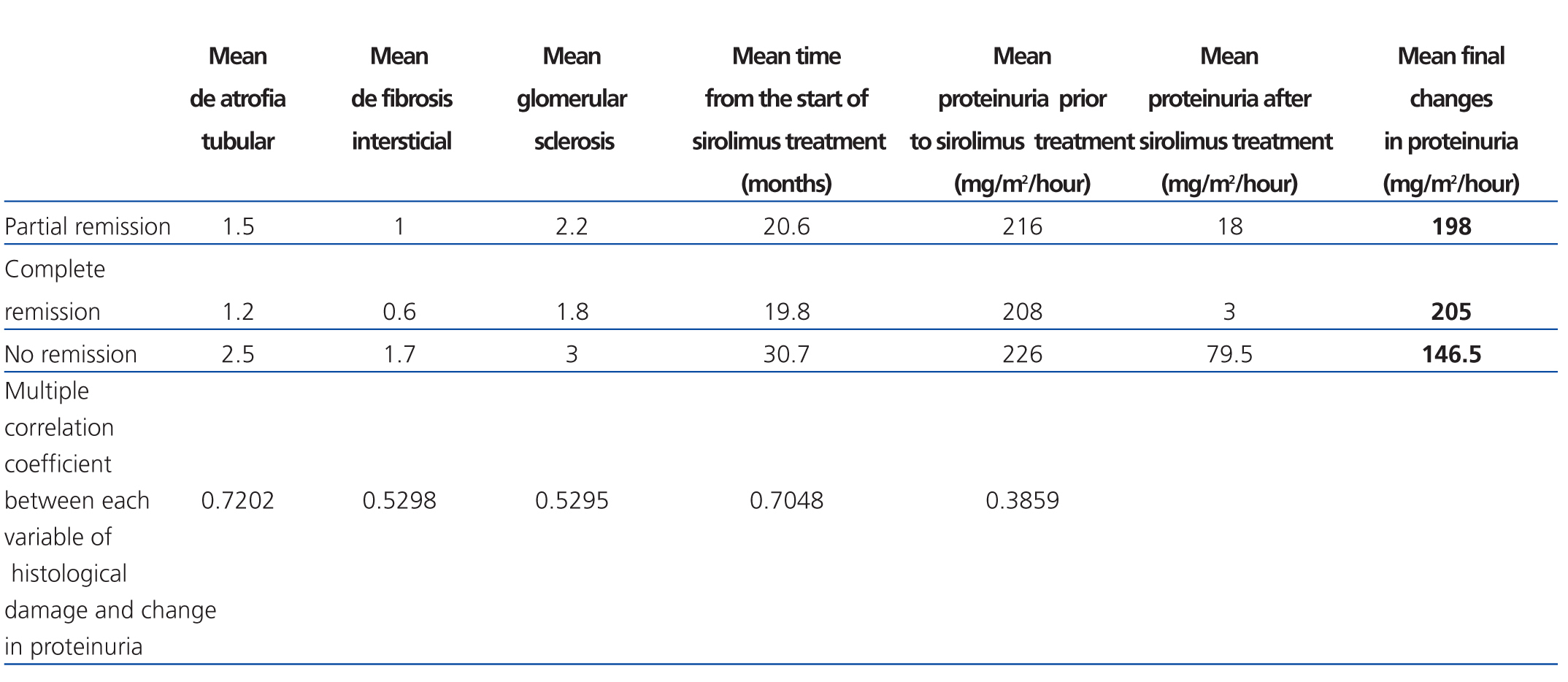
Of all the variables evaluated, the time at which sirolimus was started (a mean 21.2 months for total response patients, 25 months for partial response, and 31.5 months for no response; R2: 0.70) and tubular atrophy (R2: 0.72) had a strong correlation with variation in proteinuria for all three response types (Table 1).
On the other hand, only mild correlations were observed for interstitial fibrosis (R2: 0.52) and glomerular sclerosis (R2: 0.52), and low correlations were observed for pre-treatment proteinuria levels (R2: 0.38) (P=.01) (Table 2).
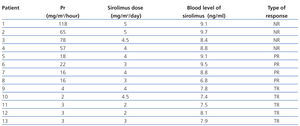
The mean dose of sirolimus was 3.6mg/m2/day (3.1mg/m2/day for total response patients, 3.5mg/m2/day for partial response patients, and 5.8mg/m2/day for unresponsive patients).
The mean EN dose was 0.18mg/kg/day (0.18mg/kg/day for total response, 0.16mg/kg/day for partial response, and 0.20mg/kg/day for no response). The mean dose of LO was 1.1mg/kg/day (1.1mg/kg/day for total response, 1.2mg/kg/day for partial response, and 1.2mg/kg/day for no response).
The mean whole blood concentration of sirolimus was 8.4ng/ml (7.7ng/ml for total response, 8.5ng/ml for partial response, and 9ng/ml for no response) (Table 3).
The following is a description of the adverse events:
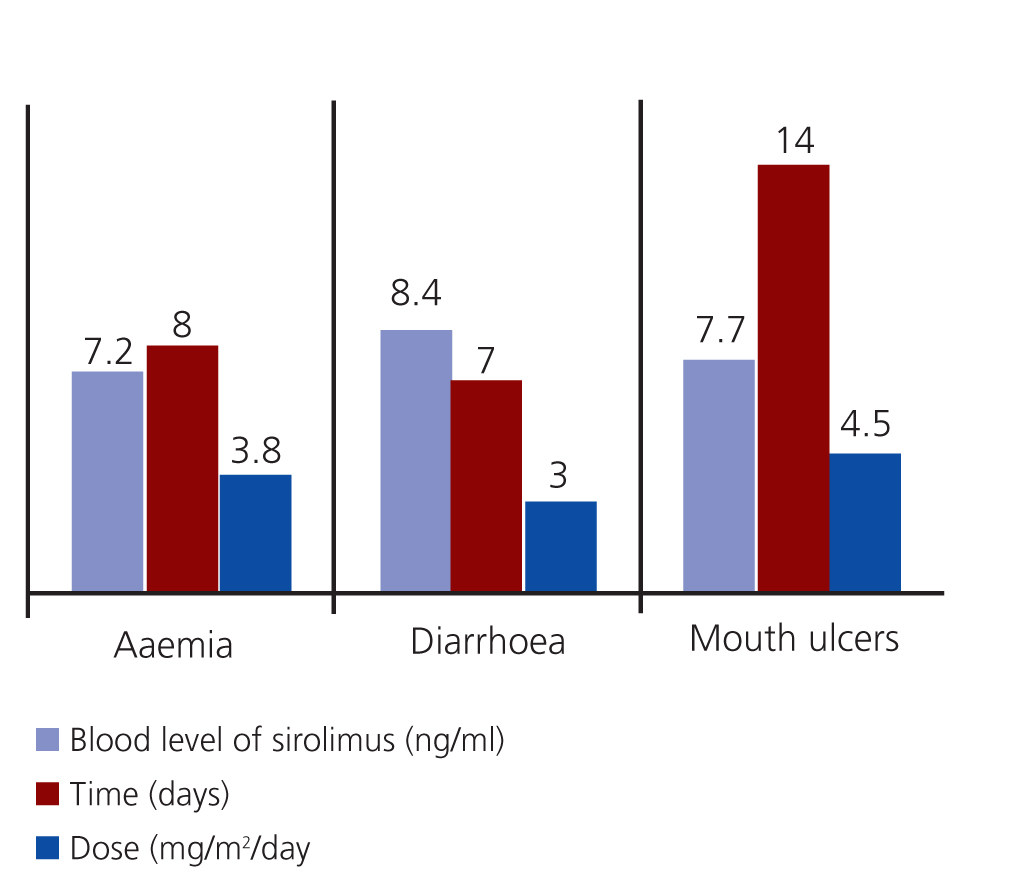
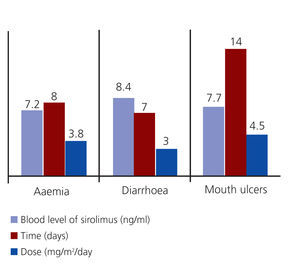
- Anaemia: Three patients. Two patients had low ferritin levels and responded well to treatment with ferrous sulphate, and the others had normal ferritin levels but inadequate transferrin saturation levels. This patient improved after receiving ferrous sulphate at 7mg/kg/day and 5mg/day of folic acid.
- Acute diarrhoea: Two patients responded well to a temporary dosage reduction of sirolimus and a diet low in fermented foods.
- Mouth ulcers: Three patients went into remission following oral rinses with sucralfate and did not require suspension of sirolimus treatment (Figure 2).
No significant differences were observed in the doses or whole blood levels of sirolimus between patients with and without adverse effects. No other adverse effects occurred that could have been correlated with treatment.
Throughout the study period, 13 patients had the following mean blood pressure values: 77±5mm Hg for total response, 74±5mm Hg for partial response, and 77±5mm Hg for no response. Serum creatinine values were: 0.8±0.1mg/dl for total response, 0.9±0.2mg/dl for partial response, and 0.8±0.1mg/dl for no response.
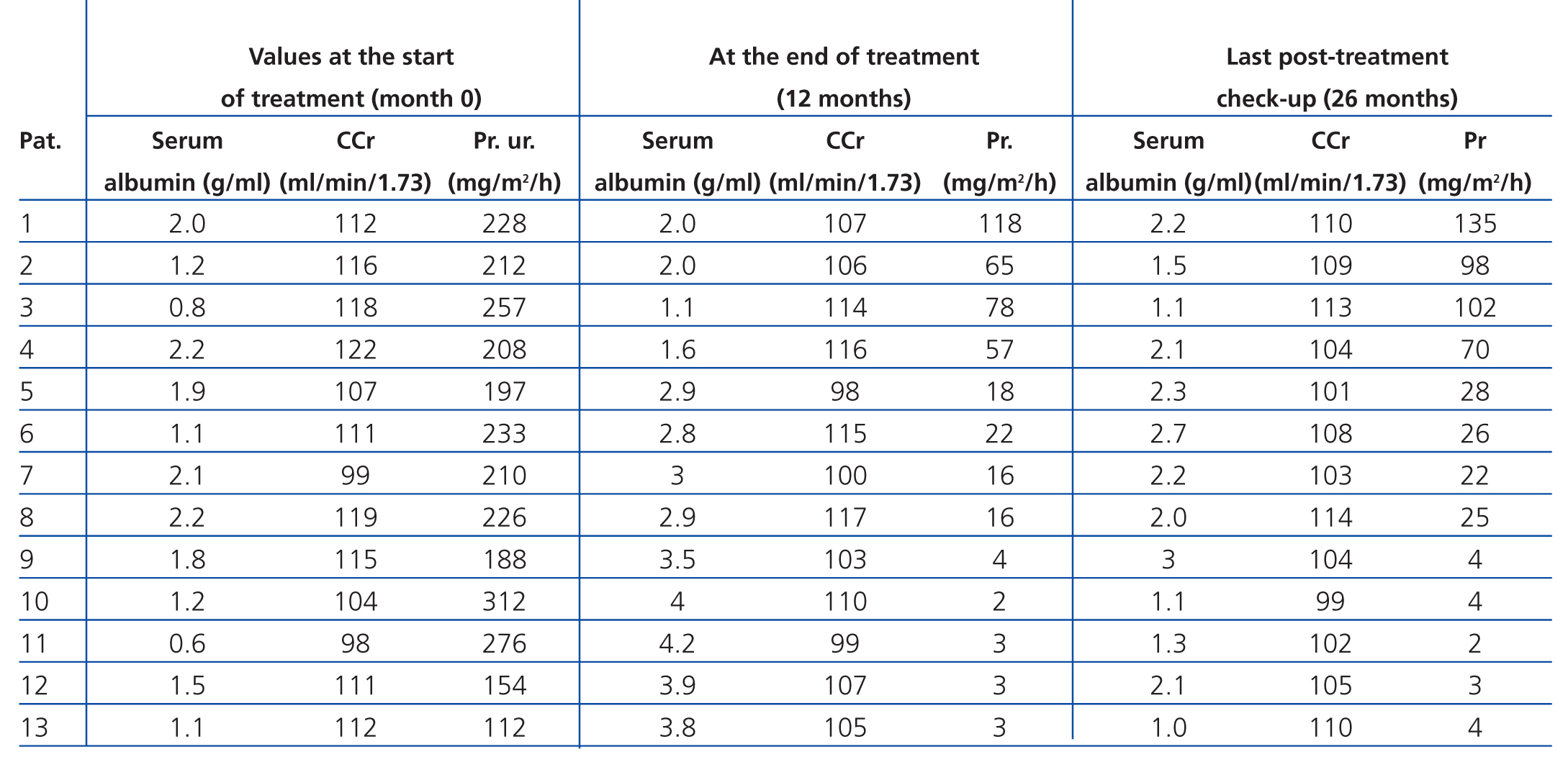
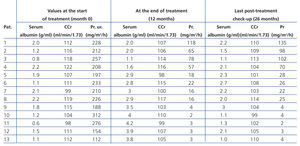
The 13 patients completed the 12 month treatment protocol, including the 4 that remained within the nephrotic range, since they still underwent a reduction in proteinuria >50% as compared to initial values. Of the 9 patients that responded to treatment, 8 had varying values of proteinuria, but had no relapses by the last check-up performed after 26 months. The 4 unresponsive patients remain within the nephrotic syndrome and have normal glomerular filtration rates (Table 4).
By the time the last control check-up was performed, none of the 13 patients had undergone another biopsy.
DISCUSSION
In our study, proteinuria values decreased by more than 90% in 9 of 13 patients. With this substantial decrease, the nephrotic syndrome went into remission in 5 patients, and the other 4 continued with significant proteinuria; even the 4 children that continued to have massive proteinuria experienced a decrease >50% from initial values. Similar results were described in a study by Tumlin,16 after 6 months of treatment with sirolimus.
The management of SRNS is a challenging health situation. Approximately 80% of patients with primary nephrotic syndrome respond to steroid treatment, and the combined use of cytotoxic drugs generally improves the rate of remission1,3; however, the remaining 20% may require other treatment regimens. This can result in very high rates of adverse effects,4,5 motivating the search for other treatment alternatives that can reduce proteinuria, even if complete recovery from renal damage is not an option. Several different alternative methods have been tested (steroids, CPM, CsA, tacrolimus, ACE inhibitors, ARB, etc.) in monotherapy and combined therapy, although results are often less than satisfactory.16,17
The use of CsA and tacrolimus-based treatment can result in14,18,19 remission rates as high as 70%, although many of the patients treated with these drugs then suffer relapses within 6 months after completing the treatment regimen. In order to avoid this large number of recurrences, treatment periods have been extended, which involves an increased risk of nephrotoxicity.18
Sirolimus is a new immunosuppressant that blocks T-cell proliferation and has a similar structure to tacrolimus, binding to the same immunomodulators without affecting the activity of calcineurin. At therapeutic doses, sirolimus blocks the proliferation of T-cells in the G1-S phase. This mechanism probably reduces structural alteration of podocytes in FSGS.19,20
Few studies have been published on the mechanism of action of rapamycin in these glomerulopathies, and the main objections are its potential long-term toxic effects.21,22 One of the most commonly mentioned sides effects is anaemia, with reduced haematocrit in 50% of the treated population. This type of anaemia can be caused by several different mechanisms, such as the drug interfering with the proliferation of primitive erythroid cells.23,24 Three of our patients developed iron-deficiency anaemia, which was effectively treated with ferrous sulphate and folic acid; we did not need to administer erythropoietin to any of our patients.
A causative relationship has been described between sirolimus and hyperlipidaemia, characterised by increased total and LDL cholesterol, apo-B100, apoC-III, free fatty acids, and triglycerides.25,26 In our study sample, hyperlipidaemia was present prior to starting the treatment protocol, which is frequently observed in nephrotic syndrome. However, considering the reduction in lipid levels observed in the group that went into remission, we were unable to establish a causative relationship between the use of sirolimus and hyperlipidaemia.
With regard to the eventual development of pathological proteinuria and renal failure due to a hyperfiltration mechanism caused by the use of sirolimus, whether in the form of haemodynamic changes (increased renal blood flow and intraglomerular pressure)27 or an unknown mechanism of nephrotoxicity, Fervenza21 reported worsening renal failure (from a pre-existing condition) in 6 of 11 patients diagnosed with FSGS, IgA nephropathy, and primary membranous glomerulonephritis, all treated with sirolimus. Letavernier described adult recipients that developed FSGS with consequent nephrotic syndrome after receiving high doses of sirolimus.28 In a study by Cho,29 0 out of 5 adult patients treated with sirolimus had a total or partial remission of nephrotic syndrome, and treatment had to be suspended due to the appearance of adverse effects (decreased glomerular filtration rate and hyperlipidaemia). In contrast, none of our patients suffered renal function deterioration (the fact that they started the treatment regimen with normal glomerular filtration rates was probably an important factor for patient evolution). Furthermore, proteinuria decreased in all of them, although not always to the same extent.
Diarrhoea is another potential adverse effect.30 In our study, three children suffered acute diarrhoea, abdominal pain, nausea, and vomiting during the treatment period, but responded rapidly to a temporary reduction in the dose of sirolimus and proper diet. The eventual appearance of mouth lesions (ulcers, glossitis, gingivitis, etc.) can occur in patients receiving sirolimus (apparently depending on the dosage). The majority of these diagnoses were made according to clinical criteria (not microbiological), and it has been proven that a reduced dose or temporary suspension of treatment can be used to minimise these effects. In addition, symptomatic treatment appears to improve the symptoms.31 Three of our patients developed mouth ulcers, which disappeared after administering oral rinses with sucralfate and did not require reducing the dose of sirolimus.
The available medical literature highlight the moment of diagnosis and an early start of treatment as factors influencing the failure of SRNS treatment.32-34 In agreement with other results, we observed that the time elapsed before administering sirolimus and the severity of histological lesions prior to treatment had an impact on the response to treatment. However, we must admit that our inability to perform genetic tests for nephrotic syndrome impeded the analysis of this important variable. We believe that the two factors are inter-related, possibly promoting the progressive development of non-immunological proteinuria, which is present in the process of hyperfiltration that damages the remaining nephrons. As such, we maintained EN and LO in combination throughout the treatment regimen. The addition of this combined therapy to sirolimus is justified in the haemodynamic and molecular mechanisms of ACE inhibitors and ARB, resulting in a decrease in the diffusion gradients and, eventually, reduced proteinuria.35,36
CONCLUSION
We believe that sirolimus is a valid treatment option for patients with SRNS, although an earlier start to treatment is probably necessary. Future studies could contribute to clarifying this issue.
Conflicts of interest
The authors affirm that they have no conflicts of interest related to the content of this article.
Table 1. Therapy and time elapsed before start of treatment with sirolimus
Table 2. Relationship between histological damage and time elapsed to use of sirolimus and variation in proteinuria
Table 3. Final proteinuria values, dosage, and whole blood levels of sirolimus
Table 4. Variation between laboratory values diagnostic of nephrotic syndrome and creatinine clearance from the start of the treatment protocol until the last post-treatment check-up
Figure 1. Variation in proteinuria values in patients that responded to treatment
Figure 2. Adverse events: time to appearance, dosage, and blood levels of sirolimus