Antecedentes y objetivos: La arteriolopatía urémica calcificante (CUA) o calcifilaxis es una enfermedad rara pero potencialmente mortal que afecta casi exclusivamente a pacientes con enfermedad renal crónica. Para su tratamiento se han empleado diferentes alternativas con resultados irregulares, siendo los bifosfonatos una de ellas. Desde 2002 iniciamos en nuestra Unidad el tratamiento con bifosfonatos en todos los pacientes con diagnóstico de CUA. Material y métodos: Se recogieron prospectivamente, entre 2002 y 2010, ocho pacientes (cuatro hombres, cinco en diálisis y tres con trasplante renal funcionante) con CUA tratados con bifosfonatos. El diagnóstico se realizó por sospecha clínica y biopsia de confirmación. Cinco pacientes con antecedentes de producto calcio-fósforo elevado, seis con antecedentes de hormona paratiroidea elevada (> 800 pg/ml), cuatro paratiroidectomizados, cinco con elevada dosis acumulada de esteroides y seis recibiendo dicumarínicos. Ningún paciente presentaba obesidad ni diabetes mellitus. Resultados: En todos los casos se constató disminución de sintomatología (dolor) a los pocos días y regresión de las lesiones entre 2 a 4 semanas tras iniciar los bifosfonatos, sin cambios en los valores séricos de calcio y fósforo. La mejoría fue más rápida en los que recibieron ibandronato intravenoso. Todos se mantuvieron en tratamiento con bifosfonatos durante al menos 6 meses, hasta que las heridas se curaron completamente. No se han observado recurrencias tras un seguimiento de entre 1 y 9 años. La función renal se mantuvo estable en los pacientes trasplantados. El tratamiento fue bien tolerado y no se observaron efectos adversos. Conclusiones: Los bifosfonatos podrían constituir una alternativa nueva y atractiva para el tratamiento de la CUA.
Background and objectives: Calcific uraemic arteriolopathy (CUA), also known as calciphylaxis, is a rare but life-threatening condition that almost exclusively affects patients with chronic kidney disease. Several therapies have been employed to treat this disease but with irregular results. We report a prospective case series of eight patients diagnosed with CUA in our unit between 2002 and 2010. Material and method: The series consisted of eight patients with CUA (including 4 men, 5 dialysis patients and 3 with functioning allografts) who were treated with bisphosphonates. The diagnosis was by clinical suspicion and a confirmatory biopsy. Five patients had a previous history of high calcium-phosphorus product, 6 had a history of high parathyroid hormone levels (>800pg/ml), 4 had undergone parathyroidectomy, 5 had a history of high cumulative doses of steroids, and 6 patients were under dicoumarin treatment. None of the patients were obese or had diabetes mellitus. Results: In all patients, progression of skin lesions stopped between 2 to 4 weeks after starting bisphosphonate therapy, with no changes in blood levels of calcium and phosphate. Improvement in pain and lesions was faster in patients receiving intravenous ibandronate. All of these patients remained on bisphosphonate treatment for at least 6 months until the wounds healed completely. No recurrences have been observed after follow-up periods between 1 and 9 years. Renal function remained stable in transplant recipients. The treatment was well tolerated and no adverse effects were observed. Conclusions: Bisphosphonates could be a new and attractive alternative to treat CUA.
INTRODUCTION
Calcific uraemic arteriolopathy (CUA), also known as calciphylaxis, is a rare but potentially life-threatening condition that almost exclusively affects patients with chronic kidney disease (CKD). The incidence of CUA among dialysis patients has been estimated at 1% annually, with a prevalence rate as high as 4%. CUA is a crippling disease with a mortality rate of 60%-80%; the main cause of death is sepsis.1,2 Despite the fact that medial calcification in large vessels is very common in CKD patients, small vessel calcification is rare. The skin’s vulnerability to CUA is probably due to its proximity to external forces, such as changes in temperature and pressure. CUA is characterised by progressive calcification of small blood vessels and the development of ischaemic necrosis of skin and soft tissues.
Given the rareness of CUA, treatment is often based on results from individual cases. Traditional treatment suggestions include debridement of necrotic tissue, antibiotic treatment to prevent or treat infection, nutritional support, correction of biochemical parameters,2 parathyroidectomy, cinacalcet, sodium thiosulphate (STS) and bisphosphonates.3-13 Bisphosphonates inhibit osteoclasts and bone resorption and are used in treating osteoporosis, Paget's disease, multiple myeloma, and tumour-induced hypercalcaemia. In animal studies, bisphosphonates have been shown to have a beneficial effect on the prevention of arterial calcification.3 Because of these recent observations, bisphosphonates were recently introduced as CUA treatment, with good results.9-13 After undertaking preliminary studies, our unit began using bisphosphonates in 2002 as a treatment alternative for all patients with CUA. We present our series of CUA cases since 2002, all of which were successfully treated with bisphosphonates.
MATERIAL AND METHOD
Study population
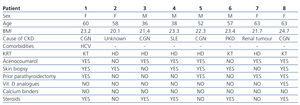
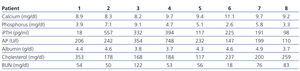
Prospective study of the 8 patients diagnosed with CUA in our unit between 2002 and 2010. They included 4 men and 4 women with a mean age of 61±7 years. Five were on haemodialysis (3 following kidney graft loss) with time on dialysis ranging between 2 and 20 years. The other 3 had functioning kidney grafts (durations between 1 and 5 years). Demographic characteristics and risk factors are summarised in Table 1, and initial laboratory results are summarised in Table 2.
Relevant data: Previous history of high calcium-phosphorous product in 5 patients (75-157mg2/dl2), previous history of severe secondary hyperparathyroidism (>800pg/ml) in 6 patients (4 had undergone parathyroidectomy), history of high cumulative doses of steroids in 5 patients, and 6 patients undergoing treatment with dicoumarin derivatives (Sintrom®) for a number of reasons (heart valve, atrial fibrillation or severe vascular access thrombosis problems). Only one patient had hepatitis C (HCV) and none were diabetic or obese. All of the patients had purple ulcerous skin lesions with a necrotic centre, erythematous edges and livedo reticularis in the entire area. The lesions were located on the inner thighs in 6 patients and in the tibial area in 3 patients (one had lesions in both locations). Diagnosis was based on clinical suspicion and a confirmatory biopsy was performed in 6 patients.
Treatment with bisphosphonates
All patients were treated with bisphosphonates. The first (patient 1) received oral alendronate 70mg/week; 4 patients (patients 2, 3, 4 and 5) received oral risedronate 35mg/week, and the last 3 (patients 6, 7 and 8) received intravenous ibandronate 6mg (1 dose) followed by a second 3mg dose after 15 days, followed by oral ibandronate 150mg/month during 6 months.
In patients on dicoumarin treatment, we decided to maintain the anticoagulant therapy for two reasons: to prevent thrombosis-related problems and determine whether the effect on calciphylaxis was due to the bisphosphonates alone.
Follow-up
In all patients, bisphosphonate treatment was maintained until all lesions had healed completely during at least 6 months. During the follow-up period, blood values of calcium, phosphorous, alkaline phosphatase and intact parathyroid hormone (iPTH) (Diasorin®) were measured monthly.
In the last 3 patients who received a second dose of ibandronate at 15 days, calcium levels were also measured prior to administrating that dose. Renal function was also measured in the patients with functioning allografts.
RESULTS
In all cases, skin lesions stopped progressing after administration of bisphosphonates. After 2-4 weeks, the edges began healing and the size of the wound diminished. Gradual decrease in pain was recorded after 2-5 days. The decrease in pain and wound size was noted more quickly in patients treated with intravenous bisphosphonates. No other drugs were used (vitamin D, cinacalcet, phosphate binders) and previous treatment and dialysis regimes were not modified.
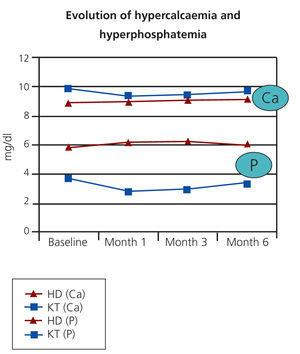
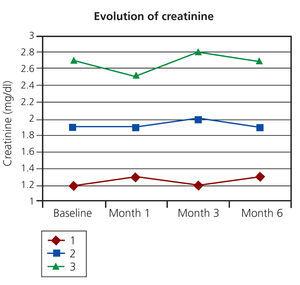
After follow-up periods ranging between 1 and 9 years, there have been no recurrences in any of the patients or death. After administration of bisphosphonates, no significant changes were reported in blood calcium and phosphorus levels in any of the cases (Figure 1). Similarly, there were no changes in iPTH or alkaline phosphatase. In patients with a functioning kidney transplant, renal function remained stable (Figure 2). The treatment was well tolerated in all cases and no relevant adverse effects were observed.
DISCUSSION
CUA develops mainly in patients with stage 3-4 CKD, in both dialysis and transplant patients. The incidence of CUA seems to have increased in recent years; the reason for this is unknown, but it could be due to better record-keeping. It occurs in up to 4% of patients on dialysis.1,2
CUA should be suspected in the presence of very painful, necrotic cutaneous ulcers in a patient with long-standing CKD. In the beginning other cutaneous manifestations are present, such as indurated plaques or livedo reticularis, sometimes accompanied by palpable deposits of subcutaneous calcium. These lesions are usually located proximal (trunk) or distal (limbs), especially along the inner thigh.1
Simple high-sensitivity x-ray can reveal calcification of small blood vessels, but the diagnostic gold standard is still a skin biopsy of the lesions, despite the risk of spreading the ulcer. The typical histological finding is calcification of the small blood vessels with intimal proliferation and intravascular thrombosis, sometimes associated with panniculitis.1 Von Kossa staining can also reveal perivascular calcium deposits. Risk factors for developing CUA include: age (patients tend to be younger), female sex, high body mass index, diabetes mellitus, longer time on dialysis, high blood levels of calcium, phosphates, calcium-phosphorus product, alkaline phosphatase and PTH. Some authors have described a potential association with HCV, and another important risk factor is being treated with high doses of steroids or with warfarin (acenocoumarol).1,2
Our series includes equal numbers of men and women, dialysis patients with very different treatment durations, and kidney transplant recipients. There was only one patient with HCV and none of the patients were obese or diabetic. Elevated blood values of calcium, phosphorus and iPTH were found in 1 patient, 2 patients and 1 patient, respectively. It is true, however, that most had a history of very high calcium-phosphorus product levels. On the other hand, most of our patients were being treated with steroids and acenocoumarol.
When CUA is diagnosed, priority treatment alternatives include normalising hypercalcaemia and hyperphosphataemia and avoiding intake of vitamin D and calcium salts. Topical treatment and beginning empirical antibiotic treatment may also be helpful.1,2 In patients with high PTH levels, parathyroidectomy was suggested as a good alternative, but results have been contradictory and risk of mortality may increase after surgery.1,2 Cinacalcet has been shown to be beneficial in some cases of CUA with secondary hyperparathyroidism, generally in combination with other treatments, such as hyperbaric oxygen and sodium thiosulphate (STS).4,8 Some cases of good response to hyperbaric treatment have been described, but this treatment is always combined with other alternatives.7
STS recently emerged as an interesting treatment option after publication of a few isolated cases and two clinical series with promising results.8 This compound seems to play a role in dissolving calcium deposits within tissues through the formation of soluble calcium thiosulphate complexes, and it may also act as an antioxidant to combat endothelial dysfunction. The most recent series, published in 2011, describes the use of STS in 6 patients. Authors reported pain relief and lesion healing in 4 patients, but 3 patients (including 2 of the 4 who responded to treatment) died less than 1 year after diagnosis.8 In addition, most of them had received at least 1 dose of IV pamidronate in the same month or the month prior to beginning STS treatment, and 2 patients were treated with cinacalcet. Only 1 of the patients did not develop adverse effects (vomiting or metabolic acidosis).
Another possible treatment alternative is the use of bisphosphonates, which are pyrophosphate analogues that are widely used in treating osteoporosis. The pyrophosphate (PPi) is a potent inhibitor of calcium and circulates in the bloodstream at levels that are high enough to prevent hydroxyapatite formation. It therefore serves as an endogenous calcification inhibitor.14,15 In particular, production of PPi by vascular smooth muscle cells may be an important defence mechanism against calcification of the vascular media. PPi has been shown to inhibit calcification of the arterial media in rats intoxicated with vitamin D.16 The effect of PPi is limited by its rapid in vivo hydrolysis. Bisphosphonates are non-hydrolysable PPi analogues which are able to inhibit vascular calcification at much lower doses.15 In recent years, bisphosphonates have been used in isolated cases as treatment for CUA, with a good response and good tolerance.9-13,17
At the pharmacological level, bisphosphonates inhibit hydroxyapatite crystallisation or reabsorption in vitro, depending on their concentration.15,18 It has been suggested that the same effect could occur in vivo. These compounds also inhibit the transformation of osteoclast precursors into mature cells. Some authors state that the similarity between smooth muscle cells and osteogenic-like cells could favour binding of bisphosphonates to the vascular wall, which would prevent ossification. Other studies have also shown that bisphosphonates reduce macrophage activity on a local level,19 and decrease secretion of pro-inflammatory cytokines.20
The administration of diphosphonate ethane-1-hydroxy-1, 1-diphosphonate and dichloromethylene diphosphonate reduced CUA lesions in rats, but its mechanism of action is still not well understood. Ibandronate, a potent inhibitor of arterial calcification, has been shown to be effective for decreasing and preventing vascular calcification in uraemic rats.3 It decreases calcium levels and reduces the ability of mineral deposits to form and grow on the arterial wall. This is probably due to its ability to inhibit the production of pro-inflammatory cytokines.
Nitrogen-containing bisphosphonates such as ibandronate can inhibit enzymes of the mevalonate pathway, thereby preventing the biosynthesis of isoprenoid compounds, including farnesyl pyrophosphate.22 Farnesyl pyrophosphate is necessary in post-translational modification of proteins, such as GTP-binding proteins (for example, Ras, Rho and Rac),23 which may be involved in the genetic expression of the OPG-RANKL complex. This may be one of the explanations for the proven effect of bisphosphonates on OPG generation, and for their contribution to healing extraosseous calcification.24
In our patients, we observed rapid improvement of pain at the lesions after beginning treatment with bisphosphonates, regardless of the type of bisphosphonate used. However, the effect was perceived more quickly with intravenous administration, which suggests an effect on the mobilisation of calcium salts in soft tissues. No significant decreases in hypercalcaemia were observed in any of our cases. However, given the potential risk of hypocalcaemia during treatment with bisphosphonates, we recommend measuring calcium levels 7-15 days after administering the dose.
In recent years (with the last 3 patients) we decided to use intravenous ibandronate as it appears to be the safest type of bisphosphonate, despite renal clearance being 50% of total clearance. No negative effects on renal function have been described. Ibandronate does not affect renal tubule cells and it does not interfere with renal tubular reabsorption or glomerular filtration.25
We are aware of the medical community’s concerns regarding the use of bisphosphonates in patients with advanced CKD, and which are mostly founded on the potential risk of causing or worsening adynamic bone disease. However, we decided to use this treatment for 2 basic reasons. Firstly, the bone-binding capacity of bisphosphonates is known to be related to remodelling activity, and in the presence of adynamic bone disease, bisphosphonates would therefore be deposited in very small quantities. The second and more important reason is that calciphylaxis is a life-threatening disease, and we prefer to run the risk of worsening a possible case of adynamic bone disease if it means reducing the morbidity and mortality rates for CUA.
In conclusion, bisphosphonates may be a new, attractive treatment alternative for CUA treatment. Based on our experience, we recommend initial administration of an intravenous dose of bisphosphonate, preferably ibandronate, in patients with a suspected case of CUA. Treatment may then be continued depending on the definitive diagnosis or patient progress. We must keep in mind that CUA is potentially fatal, and it must be treated with all the means at our disposal, including suppression of calcium salts, vitamin D and analogues, dicoumarin derivatives and precipitating factors, and by early administration of bisphosphonates or STS.
Conflicts of interest
The authors affirm that they have no conflicts of interest related to the content of this article.
Table 1. Baseline demographic characteristics and risk factors
Table 2. Baseline laboratory values
Figure 1. Serum Ca and P values after administering bisphosphonates
Figure 2. Serum creatinine values in kidney transplant patients after administering bisphosphonates