Risk factors for cardiovascular disease, including dyslipidaemia, are highly prevalent in patients with chronic kidney disease (CKD).1 Statin therapy has been shown to reduce cardiovascular events and mortality rates in patients with CKD stages G3a-G5 and it is therefore recommended.2 However, a significant percentage of patients do not achieve therapeutic targets, or experience undesirable effects.2–4 Proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i) may provide an alternative management option in these patients.
We conducted a retrospective cohort study of patients with CKD who started iPCSK9 treatment at our centre from 2016 to 2020. It was compared the total cholesterol, low-density lipoprotein cholesterol (LDL-C), triglycerides and estimated glomerular filtration rate (eGFR) at baseline, 6, 12 and 24 months. We also assessed the development and occurrence of drug-related adverse events.
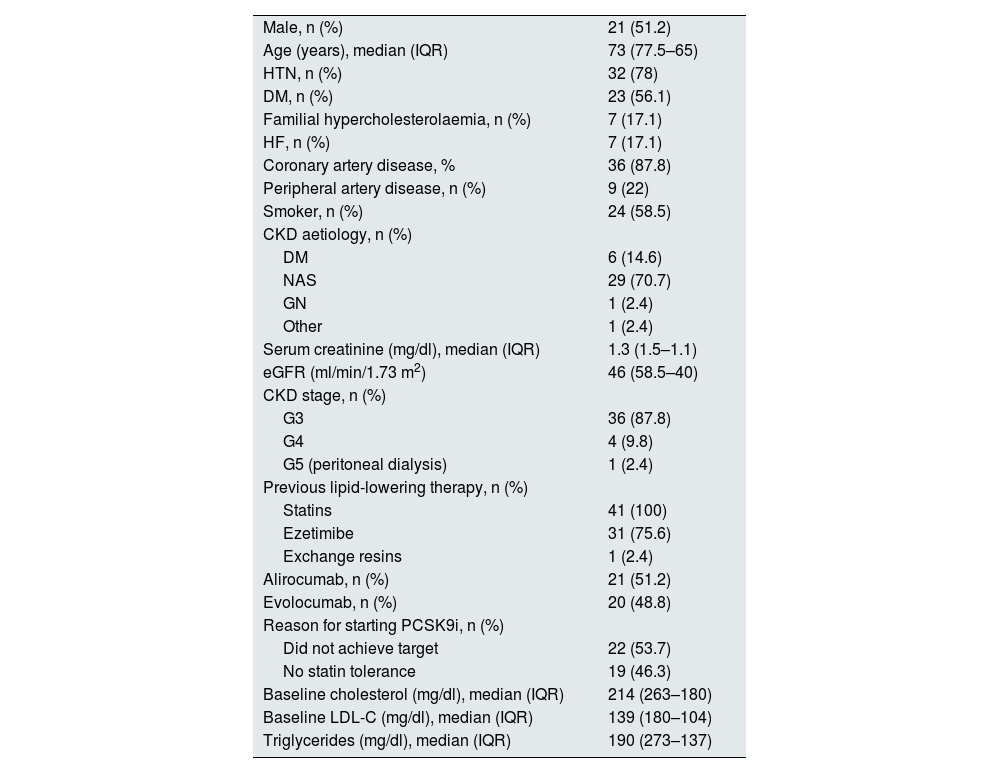
There were 41 patients included during the study period. Of these, 21 were treated with alirocumab and 20 with evolocumab. The baseline characteristics are shown in Table 1: 51.2% were men with a median age of 73 and a median eGFR of 46ml/min/1.73 m2; four patients had an eGFR <30ml/min/1.73 m2 and one was on peritoneal dialysis; 87.8% had experienced coronary events; all had previously received statins and 75.1% ezetimibe; 53.7% of patients started iPCSK9 due to failure to achieve LDL-C targets, while the remainder were due to intolerance to maximum doses of statins. Median follow-up after starting the drug was 28 months.
Baseline characteristics of all the patients included in the study.
| Male, n (%) | 21 (51.2) |
| Age (years), median (IQR) | 73 (77.5–65) |
| HTN, n (%) | 32 (78) |
| DM, n (%) | 23 (56.1) |
| Familial hypercholesterolaemia, n (%) | 7 (17.1) |
| HF, n (%) | 7 (17.1) |
| Coronary artery disease, % | 36 (87.8) |
| Peripheral artery disease, n (%) | 9 (22) |
| Smoker, n (%) | 24 (58.5) |
| CKD aetiology, n (%) | |
| DM | 6 (14.6) |
| NAS | 29 (70.7) |
| GN | 1 (2.4) |
| Other | 1 (2.4) |
| Serum creatinine (mg/dl), median (IQR) | 1.3 (1.5–1.1) |
| eGFR (ml/min/1.73 m2) | 46 (58.5–40) |
| CKD stage, n (%) | |
| G3 | 36 (87.8) |
| G4 | 4 (9.8) |
| G5 (peritoneal dialysis) | 1 (2.4) |
| Previous lipid-lowering therapy, n (%) | |
| Statins | 41 (100) |
| Ezetimibe | 31 (75.6) |
| Exchange resins | 1 (2.4) |
| Alirocumab, n (%) | 21 (51.2) |
| Evolocumab, n (%) | 20 (48.8) |
| Reason for starting PCSK9i, n (%) | |
| Did not achieve target | 22 (53.7) |
| No statin tolerance | 19 (46.3) |
| Baseline cholesterol (mg/dl), median (IQR) | 214 (263–180) |
| Baseline LDL-C (mg/dl), median (IQR) | 139 (180–104) |
| Triglycerides (mg/dl), median (IQR) | 190 (273–137) |
CKD: chronic kidney disease; DM: diabetes mellitus; eGFR: estimated glomerular filtration rate; GN: glomerulonephritis; HF: heart failure; HTN: hypertension; IQR: interquartile range; LDL-C, low-density lipoprotein cholesterol; NAS, nephroangiosclerosis.
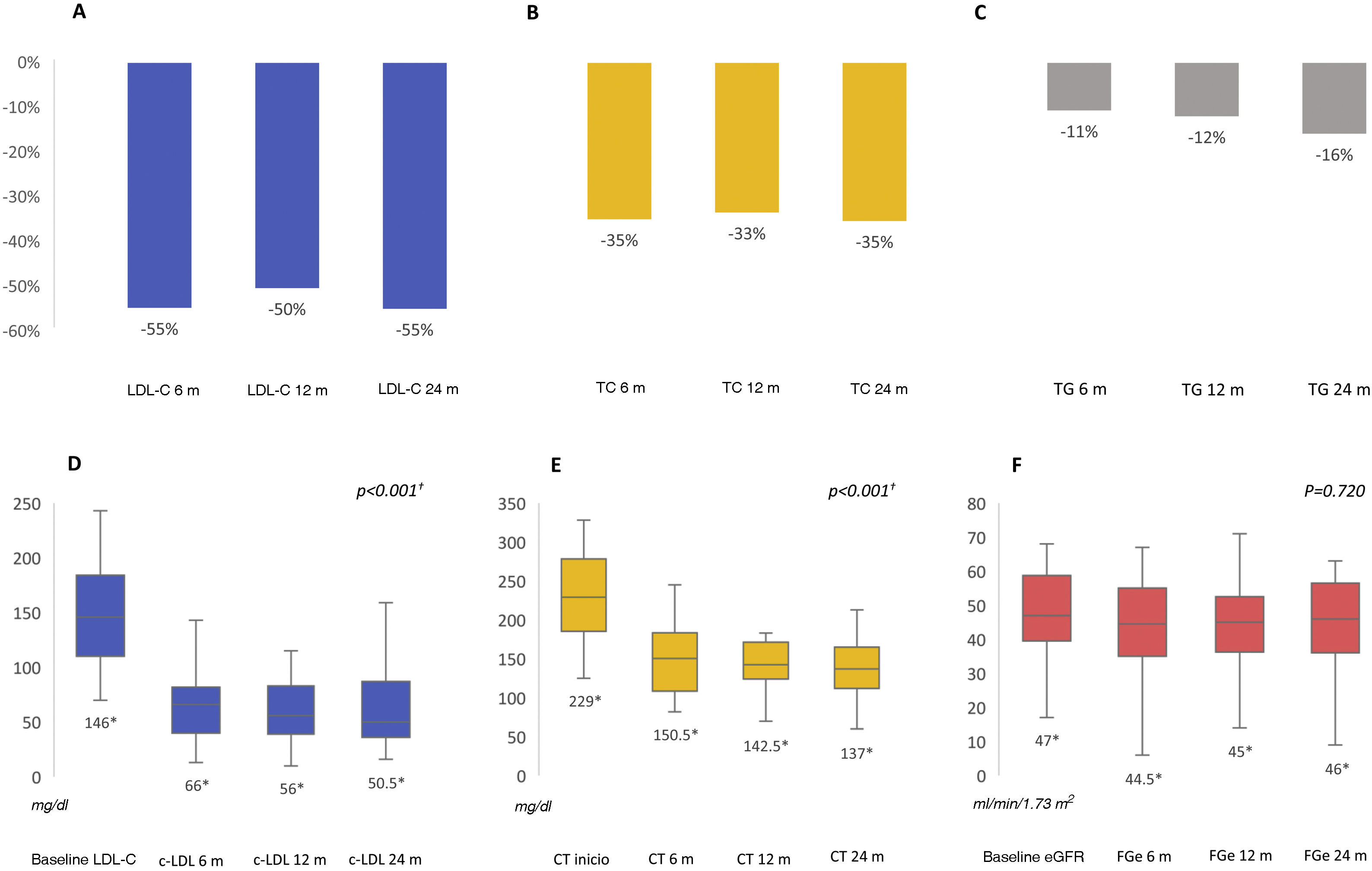
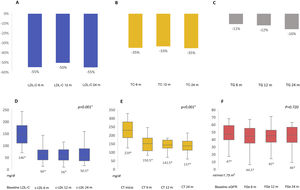
After 6 months we found a significant improvement in the lipid profile, with a reduction in total cholesterol of 35.3% (p<0.001) and in LDL-C of 54.9% (p<0.001), allowing 48.8% of patients to achieve LDL-C targets below 55mg/dl and 65% below 70mg/dl. These differences were maintained throughout the follow-up. Triglycerides were also reduced by 15.8% at 6 months (p=0.019). Renal function remained stable during the study period (p=0.720) (Fig. 1). No prescription-related adverse events or treatment drop-outs were documented, and no cardiovascular events were reported.
Percentage reduction in LDL-C, total cholesterol and triglycerides at follow-up at 6, 12 and 24 months (A, B, C); median LDL-C, total cholesterol and estimated glomerular filtration rate at follow-up at 6, 12 and 24 months (D, E, F). eGFR: estimated glomerular filtration rate; LDL-C: low-density lipoprotein cholesterol; TC: total cholesterol; TG: triglycerides.
† Comparison between baseline and follow-up at 6, 12 and 24 months. All other comparisons showed no statistically significant differences.
* Median value.
We present one of the largest series evaluating the effectiveness and safety of PCSK9i in patients with CKD in real-life. In addition, the follow-up is the longest so far described. In our experience, LDL-C was reduced by more than 50% within the first six months of treatment, enabling half of the patients to reach therapeutic targets quickly.5 This is relevant as, in patients on statin therapy, an insufficient decrease in LDL-C levels is associated with an increased risk of cardiovascular disease.4,5 A recent prospective study involving 1,886 patients with CKD found a linear relationship between LDL-C and the occurrence of cardiovascular events, and the risk is being reduced at LDL-C levels <70mg/dl.6 These patients who do not achieve therapeutic targets may benefit from the use of PCSK9i. To date, we have two retrospective series in Spain that report similar results.7,8 In our cohort of patients, the severity of CKD was greater, but it did not affect treatment safety and outcomes. One of our patients was on peritoneal dialysis, the second case reported to date.9
We can also report excellent tolerability to the drug. Renal function remained stable and there were no adverse events or withdrawals from treatment during the study period, demonstrating a safety profile similar to that of the general population.10 The development of new effective and well-tolerated treatments could offer benefits in reducing cardiovascular events and mortality rates in our patients. However, there are no clinical trials evaluating the use of PCSK9i in patients with eGFR ≤30ml/min/1.73 m2 and the evidence is limited, so PCSK9i should be prescribed with caution.
Our study has limitations, as it is a retrospective cohort study, with the inherent limitations of its design. It is also a small cohort and we did not have a control group. However, the follow-up time is longer than reported in previous series.7–9
In conclusion, PCSK9i are safe and effective in patients with CKD, even with eGFR ≤30ml/min/1.73 m2. They improve lipid control, maintain stable renal function and have a good safety profile. Due to the apparent benefits of these drugs in patients with CKD, we believe that clinical trials should be designed to include this population.