Las patologías reumatológicas, y en primer lugar la artritis reumatoidea (AR), siguen siendo unas de las principales causas de amiloidosis secundaria. La aparición de agentes biológicos como el adalimumab en el tratamiento precoz de la AR puede ser una alternativa eficaz para frenar el desarrollo y la progresión de la amiloidosis secundaria. No todos los pacientes responderán igual al tratamiento; debemos considerar la comorbilidad asociada, los factores de mal pronóstico para predecir la repuesta terapéutica y los posibles efectos adversos. Dentro de los efectos adversos de las terapias biológicas, hay que destacar el aumento de la tasa de infecciones letales y cuadros de insuficiencia cardíaca. Presentamos dos casos clínicos con amiloidosis renal secundaria a AR que han seguido un curso clínico diferente: nuestro primer caso tuvo una buena repuesta al adalimumab, mientras que el segundo caso evolucionó desfavorablemente después del inicio del tratamiento, falleciendo por complicaciones cardiovasculares.
Rheumatological diseases and, firstly, rheumatoid arthritis (RA) remain a major cause of secondary amyloidosis. The emergence of biological agents such as adalimumab in the early treatment of RA can be an effective alternative to stop the development and progression of secondary amyloidosis. Not all patients will respond the same way to treatment; we must consider associated comorbidity, the poor prognosis factors for predicting therapeutic response and possible adverse effects. In the adverse effects of biological therapies, there has been an increase in the rate of lethal infections and congestive heart failure. We present two cases with renal amyloidosis secondary to RA who had a different clinical course: our 1st case had a good response to Adalimumab while the 2nd case evolved unfavourably after treatment, and died from cardiovascular complications.
INTRODUCTION
According to the latest Glomerulonephritis Registry from S.E.N., secondary amyloidosis (AA) has an annual incidence of around 8% in renal biopsies performed to patients >65 years. AA amyloidosis is characterised by extracellular deposits of fibrillar A proteins derived from a plasma precursor that increases with inflammatory stimuli. Renal involvement is common and rheumatic diseases are the main causes, rheumatoid arthritis (RA) being first on the list.2 We present 2 clinical cases of renal amyloidosis secondary to RA that took different clinical courses.
First Case
Female patient, 57 years old, who after 8 years of evolution of seropositive RA has a 5g/24h proteinuria, plasma albumin: 3.1g/dl, plasma creatinine: 1.3mg/dl and an immunological study with no significant alterations. Besides a grade I hypertension, the patient had no more clinical history. She needed oral corticosteroids at low doses due to joint outbreaks. There is no evidence of previous treatment with gold salts, or methrotrexate (MTX), or abusive ingestion of non-steroid anti-inflammatory drugs. A biopsy of abdominal subcutaneous fat was performed. We identified focal deposits, periadnexal in subcutaneous cellular tissue and AA amyloid deposits in the vascular dermis wall.
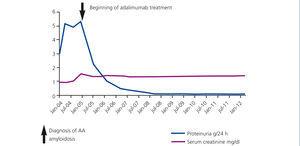
We established the diagnosis of AA amyloidosis secondary to RA. Antiproteinuric treatment was initiated in association with angiotensin converting enzyme inhibitors and of angiotensin receptor antagonists. It was administered in tolerable full doses, but with little control over proteinuria. It was decided then to use treatment with biological agents: adalimumab (Humira®) to control the activity of RA. After a year of treatment, proteinuria progressively diminished until it completely disappeared (Figure 1).
Second Case
Female patient, 78 years old, diagnosed with long term RA with joint activity and a history of stage 3B chronic kidney disease with a multifactorial aetiology in a context of dilated cardiomyopathy with preserved systolic function, mitral and aortic prostheses, moderate tricuspid regurgitation and permanent atrial fibrillation. She displayed proteinuria of 4g/24h, persistent microhaematuria, albumin of 1.7g/dl and a negative immunological study. The patient followed MTX treatment with 10mg/day, prednisone 5mg/24h. We added low-dose enalapril, which was removed after poor haemodynamic tolerance and renal function impairment. In the presence of nephrotic syndrome, we requested subcutaneous fat biopsy, which revealed the presence of deposits suggestive of AA amyloidosis.
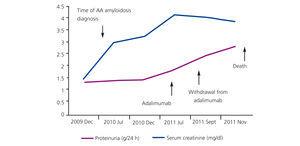
Treatment was initiated with adalimumab (Humira®) associated with low-dose MTX (7.5mg/week) and prednisone 5mg/day. During follow-up, the patient had low blood pressure and progressive renal deterioration, plus multiple episodes of volume overload and cardiac decompensation baseline (Figure 2). In November 2011, she suffered a cardiac arrest and recovered after advanced cardiopulmonary resuscitation. The evolution in the ICU was very unfavourable. The patient presented cardiogenic shock refractory to vasoactive drugs. Severe diastolic dysfunction was observed, and multiple irreversible organ failure in terminal circumstances; death was unavoidable.
DISCUSSION
Renal amyloidosis can show up as microscopic haematuria and nephrotic range proteinuria with chronic or rapidly progressive kidney failure.2-4 It is less frequent to find acute renal failure and tubulointerstitial isolated manifestations.5 Amyloid deposits are in the glomerulus; at other times, they predominate in vessels or tubules with some sort of tubular dysfunction. AA prognosis worsens with renal and/or heart involvement. The main prognosis factors are albumin, creatinine, levels of serum amyloid A and 24-h proteinuria values. The higher these values, the higher the mortality due primarily to infections, cardiovascular problems and intestinal haemorrhage and perforation.6
Treatment of AA amyloidosis is aimed at attenuating the inflammatory stimulus through properly controlling the course of the responsible disease. The goal during the first phase of treatment is to avoid the deposition of fibrillar proteins and to reduce the synthesis of its precursor: serum amyloid protein (SAA). Monitoring SAA levels is a useful parameter of patients follow-up since high levels of it (above 10mg/l) are associated with increased mortality and poorer renal function.2,7
Tumour Necrosis Factor Alpha Antagonists
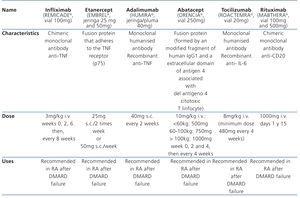
In recent years, there has been a widespread use of biological agents (tumour necrosis factor inhibitor [anti-TNF]) in the early treatment of moderate to severe RA, alone or in combination with MTX (Table 1). The first anti-TNF in the market was infliximab (Remicade®), followed by etanercept (Enbrel®) and adalimumab (Humira®). The all have anti-inflammatory properties, capable of attenuating the inflammatory activity of RA, improving symptoms and decreasing the progression of radiological damage. Their effectiveness is increased when they are associated with MTX. The superiority of one over the other has not been shown and the lack of response to one of them does not imply a lack of response to another.8-10
Studies have shown, after starting treatment with anti-TNF, a significant reduction in amyloid deposits in control gastroduodenal biopsies.11 The use of anti-TNF level helps reduce renal glomerular inflammation and proteinuria, which in turn decreases the synthesis of the inflammatory mediators responsible for changes in glomerular albumin permeability.7 Consequently, its early use in renal amyloidosis may favour a better control of the proteinuria9 but its effectiveness is unknown in advanced stages of renal and/or cardiac amyloidosis.
Adalimumab
Adalimumab is a humanised class IgG 1 monoclonal antibody specifically directed against TNF-α. Its main mechanism of action is blocking soluble and membrane TNF-α, inducing apoptosis of activated T lymphocytes and monocytes. It also inhibits the production of proinflammatory cytokines, such as interleukins (IL) IL-1 and IL-6.8 Adalimumab provides a better dosing system: once every 2 weeks (Table 1), avoiding the intravenous path (infliximab) or a greater number of doses per week (etanercept). Moreover, unlike infliximab, adalimumab may be administered in monotheryapy, without MTX.
In reference to our patients, the cases had a very different evolution. The first patient had minor renal involvement, implying a better prognosis, and presented a better response to biological agents. Our second patient had poorer renal function, higher comorbidity and continued on MTX treatment to control her symptoms. We decided a therapeutic change to adalimumab, we reduced the dose of MTX to avoid further nephrotoxicity and assessed the possibility of complete withdrawal according to patient's evolution. The patient initially had a preserved systolic function. However, later she presented a severe diastolic dysfunction reflected by possible cardiac involvement secondary to amyloid deposition, although adalimumab may have negatively influenced her cardiopathy.12 Treatment with adalimumab must be discontinued if a patient develops a basal cardiac decompensation or congestive heart failure (HF). It is contraindicated in patients with moderate to severe HF. It is advisable, before indicating a treatment, assessing each case individually by taking into account the associated comorbidity, the risk of opportunistic infections and prognostic factors, especially heart and renal factors.13-15 All patients being treated with anti-TNF must have completed the hepatitis B vaccination and receive the pneumococcal and influenza vaccine. They will have an annual tuberculin test.15
Therapeutic alternatives in amyloidosis secondary to rheumatoid arthritis
Tocilizumab
Tocilizumab is a recombinant monoclonal antibody directed against IL-6, the latter plays a role in the production of SAA. Cases have been described where the tocilizumab, in combination with MTX, has been very effective in treating amyloidosis secondary to RA. It is capable of reducing circulating levels of SAA16 and amyloid deposits in gastrointestinal biopsy even until their full disappearance.17
Rituximab
Rituximab (RTX) is a chimeric monoclonal antibody anti-CD20. We have described a potential therapeutic effect of RTX in the treatment of AA secondary to RA.18 Treatment consists of two infusions of 1000mg RTX in combination with MTX.
Calcineurin inhibitors (tacrolimus) and azathioprine
Tacrolimus has an attenuating effect on the inflammatory response of RA, decreasing the synthesis of inflammatory cytokines, particularly TNF-α, IL-2 and interferon γ. In a prospective study of patients who had a poor response to other biological agents, low-dose tacrolimus (2mg/day) was associated with anti-TNF. After 4 weeks, we observed a significant decrease in inflammatory activity, reaching its maximum efficiency at 54 weeks.19
On the other hand, a case amyloidosis secondary to RA has been reported where the use of azathioprine has successfully allowed control of the disease and remission of nephritic syndrome.20
Eprosidate
Finally, we mention eprodisate, a polymerization inhibitor of amyloid fibrils and their deposition in tissues. It has been tested in the treatment of AA amyloidosis with renal involvement and, after follow-up for 24 months, has been shown to slow renal function deterioration.21 We are currently waiting for the results of a second clinical trial that will end in May 2014.
CONCLUSION
AA Amyloidosis secondary to RA may respond to treatment with biological agents, such as adalumimab. Its use allows for better control of proteinuria, but we are unaware of its effectiveness in advanced stages of renal and/or heart amyloidosis. Not all patients will respond the same way to biological agents. We must establish a preliminary selection of patients, considering the associated comorbidity, poor prognosis factors for predicting response to biological agents and potential adverse effects.
We should point out that the most effective treatment of AA amyloidosis is the one able to stop promptly the evolutionary course of the disease responsible for attenuating the underlying inflammatory stimulus.
Table 1. Drugs usually used on mild to severe active rheumatoid arthritis
Figure 1. Patient 1: development of proteinuria and serum creatinine
Figure 2. Patient 2: development of proteinuria and serum creatinine