The peritoneal equilibration test (PET) allows for analysing the characteristics and mechanisms of the transport of water, electrolytes and other solutes across the peritoneal membrane. Since its initial description by Twardowsky,1 much experience has been accumulated with regard to this test and the means and ranges of peritoneal solute transport have been established for different populations (Figure 1). The main practical aim of PET is to simplify estimates of peritoneal function and facilitate the correct prescription of peritoneal dialysis (PD). Correct standardisation is an essential element for PET validation. From the initial description of the procedure2, modifications in the conditions of the test have been introduced in order to obtain more reliable and more complete information on membrane function. These modifications have mainly affected glucose concentrations in the dialysate used for carrying out the test,3-6 but also other factors, including the duration of the test itself.7 Furthermore, the development of the PET has encouraged other study strategies. For example, research groups from northern Europe have developed an alternative test lasting 24 hours, involving several exchanges in which glucose concentration in the dialysate and the duration of treatment are modified (peritoneal dialysis capacity, PDC test).8
The simplicity of the PET allows it to be used for a sequential evaluation of peritoneal function, since it can be repeated periodically whenever clinical circumstances demand it. The baseline PET, carried out in the first weeks of treatment with PD allows intrinsic functional abnormalities of the peritoneal membrane to be detected and serves as a reference point for detecting changes occurring during the course of treatment with PD.9
The PET, as with any other diagnostic test, has limitations to its validity and accuracy, which should be known and taken into account when interpreting its results. With regards to this disputed matter, I have been asked to highlight the limitations and negative elements of the PET as a method of study of peritoneal function. My approach may not, in any case, be contrary to the general validity of the PET, but will present arguments against its “sanctification”, showing its main limitations and drawbacks. I will also explain the feasibility of prescribing PD on the basis of a routine clinical data assessment that does not include the PET. Overall, I will divide my argument into four issues:
1. Does it make sense to maintain classical PET glucose at 2.27/2.5% as a reference?
2. What are the major limitations and drawbacks of the PET?
3. Can PD be prescribed without the assistance of the PET?
4. What is the real value of comparing the results of sequential PET studies?
1. MAINTAINING THE CLASSIC PERITONEAL EQUILIBRATION TEST WITH GLUCOSE AT 2.27/2.5% AS A REFERENCE
From Twardowsky’s description of the classical PET, four peritoneal transport categories were established, based on the saturation of creatinine and glucose uptake (high, medium-high, medium-low, low).1 These terms have been redefined in recent years in a simpler categorisation, as fast, medium and slow transport, although the classical terminology is still widely accepted. Creatinine and glucose have a similar molecular size and behaviour, though inverse and not identical with respect to the peritoneal membrane. It has been assumed, without much evidence, that glucose uptake would show a strong inverse correlation with ultrafiltration (UF). The result was the mistake, common for years, of assuming that the transport of creatinine was, in turn, a reliable inverse marker of UF capacity. Neither the current knowledge of the physiology of the membrane nor the sizeable amount of evidence accumulated support this claim whatsoever. UF capacity shows statistically significant correlation with the transport of small solutes, but the degree of association is totally insufficient to extrapolate one variable to the other.9 If we want to know the capacity of UF, it must be estimated in a specific and standarised manner. This is the basis of the proposed modification to the Classic PET, which has been based on glucose 2.27/2.5% for over a decade, and the use of glucose solution at 3.86/4.25% is increasingly being recommended for PET. This solution provides results that are comparable to those for 2.27/2.5% in terms of transport of small solutes3-6 and, by allowing greater UF, it provides more reproducible estimates of this variable. The International Society for Peritoneal Dialysis (ISPD) has adopted a UF of less than 400ml for the PET at 3.86/4.25% as an indicator of peritoneal UF failure.10
The transport of water across the peritoneal membrane occurs along two main routes that can be studied separately using the PET. This is possible because, during the first 60-120 minutes of the exchange, the water transport occurs at more or less 50% through small pores (along with electrolytes and other solutes) and intracellular water channels (aquaporins) (a tract exclusive for water). Knowing the functional condition of both tracts provides information with significant clinical implications, especially for patients with UF problems. The classical way to deal with this phenomenon is the sodium sieving analysis, that is, the decrease in sodium concentration in the dialysate at the end of the first hour of the PET, from which we can extrapolate the fraction of water which has been transported in this period that is free of electrolytes and, therefore, through the aquaporins. Although this phenomenon can be analysed with the classical PET at 2.27/2.3%,11 the PET at 3.86/4.25% provides more discerning data on it.
In recent years, different variants of PET have been designed to fine-tune peritoneal water transport analysis. Thus, La Milia has proposed the ‘mini-PET’, with glucose at 3.86/4.25% with a duration of one hour.12 With this test, sodium sieving, free water transport and the transport of water through small pores can be estimated more accurately. However, comparison of saturation rates of small molecules with those obtained in the PET with a duration of four hours shows a poor correlation and as such, performing the mini-PET does not eliminate the need to carry out the classic 4-hour PET. Performing a modified PET, with total drainage after one hour and reinfusion to complete the 4-hours, allows unification of both tests.13
The capacity of the peritoneal membrane to generate UF in response to different glucose concentrations (osmotic conductance) can be explored using the double mini-PET technique, which involves performing two mini-PETs (1 hour), the first at 1.36/1.5% and the second at 3.86/4.25%.14 When this test is combined with subsequent reinfusion of dialysate to complete a 4-hour dwell, we talk about UNI-PET. The latter test increases duration by 2 hours with respect to the classic PET, and as such is probably less applicable in normal clinical routine.7
During the course of a peritoneal exchange, re-uptake of dialysate occurs through the peritoneal lymph nodes at a relatively constant rate, which none of the above PET variants calculates. Adding a volume marker (for example, Dextran 70) to the dialysate used in a PET allows this parameter to be calculated,15 but this variant is not applied in clinical routine.
The calculation of the apex-time (the point at which creatinine saturation curves of creatinine and glucose uptake cross in a PET at 3.86/4.25%) may be useful in calculating the optimum intraperitoneal period of dialysate in automatic PD (APD).16
The PDC test allows estimation of the area of the membrane, the flow through the large pores and dialysate re-uptake by a test lasting 24 hours of multiple analytical determinations. This complexity and the need for specific software make its use less widespread than PET in normal clinical practice.
Overall, the development of multiple versions of the classic PET confirms that the information obtained may be insufficient for understanding the peritoneal transport characteristics in the clinical setting. The correct characterisation of the causes of UF failure and the possibility of early diagnosis (including prevention) of sclerosing peritonitis are underlying goals of these initiatives. Which of these tests is best for clinical routine? As a “multipurpose” test, perhaps the PET at 3.86/4.25% for 4 hours with total drainage and reinfusion at 60 minutes is the test that provides the most comprehensive information on peritoneal transport of water and solutes without adding complexity, a key issue in our daily clinical practice. The double mini-PET provides additional information that is unquestionably useful in cases in which there is a UF deficit.
2. LIMITATIONS TO THE ACCURACY OF THE PERITONEAL EQUILIBRATION TEST
The accuracy of the PET as an estimator of peritoneal transport is limited by three types of factors related to the proper functioning of the peritoneal catheter, the patient’s clinical situation and possible inaccuracies in sample processing. In practice, the major issue is the need for an optimal peritoneal drainage mechanism and it must be so in at least two successive exchanges: the pre-test and the PET test itself. The presence of a significant residual volume at the start of the test increases estimates of peritoneal transport of solutes and, if drainage during PET is complete, it results in an overestimation of UF capacity. Furthermore, if drainage during the PET is incomplete, UF capacity will be underestimated.
The clinical status of the patient may potentially affect the results of the PET, although the clinical significance of this interference is unclear. In practical terms, the states of hyper-or hypovolaemia and, above all, hyperglycaemia, are those that can compromise solute transport and UF estimates.
Sample processing is another major source of errors in the PET estimates. Although the test is standardised in great detail, the risk of errors in daily practice is high. For example, if the mix of the dialysate at the time the sample is extracted is incomplete, the test results may be significantly altered. Moreover, a delay in the processing of samples can decrease the concentrations of urea and creatinine. Although most laboratories automatically make a correction of the creatinine figures with the glucose concentration in the liquid, these are estimate formulas, and in case of not being applied, a mean increase of 0.5mg/dl occurs in creatinine levels for each 1000mg/dl of glucose.
The need to adjust the volume of dialysate used in PET to the patient’s body size is often overlooked.17 Ignoring this factor leads to an underestimation of the transport of small molecules in large subjects and an overestimation in small subjects. The European working group on best practices in PD18 recommends infusing the same volume as the patient usually uses, which is also better adjusted to what actually happens in day to day treatment.
Lastly, there are some minor drawbacks to the implementation of the PET, which must be taken into account:
-Glucose overload, with a mean uptake of 38% of infused glucose. This is, as a mean, 17 grams in the classic PET, 29 grams in the modified PET with glucose at 3.86/4.25%. This factor has more potential importance for diabetic patients, who may show hyperglycaemia during the test.
-Potential risk of haemodynamic instability in patients with high rates of UF during PET 3.86/4.25%. It is unusual, but not exceptional, that a patient may require intravenous volume replacement.
-The PET involves in-patient use of the PD system with a low, but not negligible risk of disconnect and peritonitis.
-The economic cost of the classical PET is low, but it involves a time commitment both for nurses and the nephrologist in charge, in addition to the cost of carrying out a blood sample and five dialysate tests.
3. CAN PERITONEAL DIALYSIS BE PRESCRIBED WITHOUT THE AID OF A PERITONEAL EQUILIBRATION TEST?
The answer to this question is simple: of course it can. It is true that the usual prescription of PD is based on body size (direct relationship), residual renal function (inverse relationship) and peritoneal transport characteristics. Of these three factors, the third is the one with a less direct influence on practical decisions. In fact, the initial requirement is not taken into consideration since it is unknown at this time. Many nephrologists believe that knowledge of peritoneal transport type is not necessary for prescribing PD, preferring the trial and error method (prescribe empirically and then adjust according to adequacy results). In addition, the transport effect of the type of transport is lower in most cases, since approximately 80% of patients present average permeability (Figure 1).
The European Dialysis and Transplant Association (ERA-EDTA) published in 2010 its recommendations for the initial prescription of PD.18 They advocated the convenience of estimating the UF capacity of each patient from the exchanges made during the training phase. If the patient has a high UF capacity with low dialysate glucose concentration after a more than 4 hours permanence, he/she will surely be a slow transporter, and will benefit from higher volumes and long dwells. Conversely, if the patient has limited UF capacity (negative UF after 1,36%/1,5% glucose exchanges of less than three hours), he/she will probably be a fast transporter, and short duration dwells (APD) with higher concentrations of glucose should be scheduled, using icodextrin for the long dwells.
In non-complicated prevalent patients, routine monitoring does not require as much PET as it does clinical evaluation and adjustment controls. The common clinical problems are typically addressed, in this way from the outset. For example, if the patient shows signs of metabolic deterioration or underdialysis, the first step is to estimate residual renal function and peritoneal clearance. If signs of overhydration appear, we must first consider factors not related to the membrane and the degree of compliance with diet, a rapid drop in residual diuresis or peritoneal catheter malfunction. The patient that shows inadequate UF capacity is the main potential beneficiary of the PET which will help to correctly categorise the nature of the problem. However, the interpretation of results in these patients is often confused because the limitations of the PET are more obvious when there is only one estimate. Often, trial and error (modify the volume, duration and concentration of changes, test icodextrin) is used. The practical guidelines of the Canadian Society of Nephrology for PD do not recommend the routine use of PET for PD prescription.19,20
4. UTILITY OF THE PERITONEAL EQUILIBRATION TEST TO MONITOR THE DEVELOPMENT OF PERITONEAL FUNCTION
The existence of an anatomical and functional response of the peritoneal membrane to sustained contact with nonphysiological dialysis fluids has been known for years.21,22 More recent studies have revealed the mechanisms by which the exposure to these solutions alters the membrane, including the loss of the integrity of the mesothelium and the development of epithelial-mesenchymal transition phenomena on an interstitial level with extensive fibrosis and in some cases, neoangiogenesis. Likewise, a characteristic vascular lesion occurs. These structural changes display a functional correlation which most commonly expresses as is increased peritoneal transport of small solutes and progressive loss of UF capacity. However, this anatomo-functional correlation is far from unambiguous, as structural changes are almost universal, whereas the majority of patients maintain a relatively stable functional status. The reasons for this discrepancy are not entirely clear, but one factor to consider is the imprecision of the methods used to detect alterations in peritoneal function in clinical practice including, prominently, the PET.
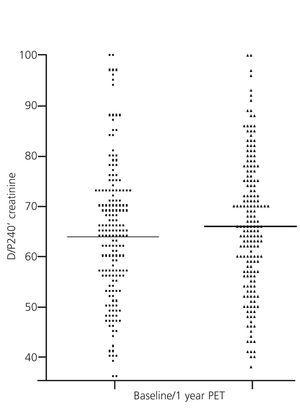
The PET is the method currently accepted for detecting changes in peritoneal function throughout PD treatment. This monitoring is conducted on a regular and systematic basis in some centres, while in others it is only used in the presence of warning signs (e.g., decline in UF capacity). However, the inaccuracy inherent in the PET hampers reproducibility, and limits the comparison of studies over time. Nearly two decades ago, Davies et al.23 showed significant variability estimates in peritoneal transport of small solutes by PET, comparing it with a control baseline test carried out at 6 months. In 23% of cases, discrepancies in over 20% creatinine saturation without any apparent cause were observed. In this context, it is not useful to try and classify patients into very limited peritoneal transport subgroups, which helps explain the current trend of identifying only patients with extreme values, leaving a wide area of average transport. In a comparative study of the results of the PET with 2.27/2.5% and 3.86/4.25% glucose, our group found no overall significant differences, but, remarkably, 47.7% of the patients changed transport category between tests in a in a relatively short period of time.12 The variability was much less when all average transporters were grouped. Our group has also compared the results of the baseline PET (second month) with those obtained at the end of the first year in a large population of PD patients and found no apparent changes (Figure 2). However, subgroup analysis showed that patients with extreme permeabilities tended to converge toward the mean (Figure 3), a behaviour that could suggest the existence of a non-specific bias due to inaccuracy in estimates, rather than a pattern of peritoneal membrane response. Other studies have shown similar results, with an evolution towards average values during the first year in fast transporters mean, with a reduction in the permeability of small molecules and an increase the UF capacity.24 Lastly, several studies, some of them from Spain, have shown that the baseline characteristics of peritoneal transport are unable to predict the late behaviour of the peritoneal membrane.25
FINAL REMARKS
The main contribution of clinical peritoneal function tests is the information that they provide about UF capacity and, if this fails, the possible mechanisms. For this purpose, 3,86/4,25% glucose-based PET, with modifications oriented to analyse free water transport, provides clear advantages over the classical 2,27/2,25% test.
The results of the PET are also helpful for PD prescription, and are indispensable if prescription programmes are used, but it is clear that PD can be correctly prescribed, without a PET, based solely on clinical assessment and adequacy results.
It must be remebered that an isolated PET has a only a relative accuracy for estimation of peritoneal transport of water and solutes. The main reason is its dependence on an optimal peritoneal drainage during two consecutive exchanges. For the same reason, the results of serial PET studies should be evaluated wiyhin a comprehensive clinical and laboratory assessment.
For the same reason, the results of consecutive PET should be compared within a comprehensive clinical assessment scheme.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Figure 1. Examples of peritoneal transport categories distribution in peritoneal dialysis populations in Europe (Hospital Universitario de A Coruña), the Americas (Canada) and Asia (South Korea)
Figure 2. Comparison of values of creatinine peritoneal transport during peritoneal equilibration tests performed at baseline and at the end of the first year of treatment (differences not significant)
Figure 3. Mean values of the baseline peritoneal transport of creatinine and its values at the end of the first year of treatment, stratified according to baseline category