When the organising committee of the VII National Peritoneal Dialysis Conference invited me to participate in this controversy in support of the usefulness of performing peritoneal kinetic analysis in standard clinical practice, my response was a firm yes. We strongly believe in the need to perform tests of peritoneal function in order to ensure appropriate prescriptions and to facilitate an early detection of functional changes that could foreshadow the appearance of structural lesions with pathological significance that can be induced by the dialysis technique.
THE USEFULNESS OF PERITONEAL KINETIC ANALYSIS IN DETERMINING PRESCRIPTIONS
The use of the peritoneum (biologically reactive tissue that varies between individuals) as a semipermeable membrane for administering dialysis inherently implies inter-patient differences in types of transport. Several studies have demonstrated a wide range in the characteristics of baseline peritoneal transport rates, and that these rates are unpredictable based solely on sociodemographic factors, the underlying disease, or patient comorbidities.1,2 These characteristics can also change in response to long-term application of peritoneal dialysis (PD), which involves permanent exposure to non-biocompatible fluids and the risk of peritonitis as an occasional complication of this technique.3-5 Understanding the characteristics specific to each patient in terms of water and solute transport will aid in administering an appropriate prescription and optimise exposure times, thus avoiding over-exposure to osmotic agents, such as glucose and glucose degradation products (GDP),5-7 which could damage the membrane.
Should peritoneal kinetics be measured at initial levels in order to determine the optimal modality of peritoneal dialysis?
The Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines from 2006, the directives for adequacy of dialysis established by the CARI guidelines (Caring for Australasians with Renal Impairment),8 and the European guidelines for good clinical practice in PD9 all coincide in their proposal for analysing peritoneal kinetics 4-8 weeks from starting PD in order to optimise the initial prescription. However, this recommendation is not universal; in fact, the recently published Canadian guidelines10 do not consider this to be an essential step in treatment, based on the concept that patients at the start of their time on PD still retain residual renal function (RRF) and that reaching adequately negative water and sodium balance and Kt/V values is not difficult, both on continuous ambulatory PD (CAPD) and automated PD (APD). The authors argue that when RRF is still present, the choice of PD modality is based more on criteria focusing on quality of life and patient preference than the type of transport presented by the patient; however, in a recent letter sent to Peritoneal Dialysis International, the argument is set forth that a baseline peritoneal equilibration test (PET) can be of assistance in establishing the initial prescription.11 It is also worth mentioning that the inferior prognosis associated with patients who have high initial transport values12 disappears when these patients are treated from the start with APD and/or icodextrin.12-14 As such, the diagnosis of high transport (HT) patients in initial stages could aid in establishing the indication of APD with or without icodextrin in many cases, not only because this yields improved parameters in terms of adequate dialysis treatment, but it would also improve the prognosis of the patient.
To summarise, most guidelines recommend analysing baseline peritoneal kinetics in order to provide another useful tool in the process of prescribing an adequate dialysis regimen and to evaluate the initial characteristics of the peritoneum.
What type of peritoneal kinetics should be measured in the baseline analysis?
Various tests have been described for the evaluation of peritoneal function, but no randomised and prospective studies have been carried out to determine which is best. The ideal kinetics test would measure both the capacity for transport of small solutes (diffusive capacity) and transport of water and sodium (convective transport), would be simple to carry out in normal clinical practice, and would yield reproducible and stable results.
The most commonly used test, and that most highly suggested by the majority of guidelines,8,9 is the PET with a 4-hour exchange at 2.27%, as proposed by Twardoski in 1987.15 In the context of small molecule transport, this protocol involves analysing the dialysate/plasma ratio of creatinine 240 minutes after the exchange. Based on these results, patients are classified as high, medium-high, medium-low, or low transporters, according to the groups initially described by Twardoski. The success of the PET lies in its simplicity and the fact that it is the most commonly used method in the majority of health care institutions, above all for the measurement of diffusive transport, which provides reference values for studies and comparisons with previous experiences.
However, the PET-2.27% is associated with a varying level of imprecision due to the fact that this is a semiquantitative metric (classifying patients into transport categories) with a high coefficient of variability at 10%-25%, mainly if the methods used have not been properly standardised in terms of duration of the exchange prior to measurement, infusion volumes used, body position of the patient, and the laboratory methods used. But the greatest issue with the PET-2.27% is that it does not differentiate well between the different causes of ultrafiltration (UF) failure, it only assumes that HT patients would have a lower UF rate since, in these patients, glucose would diffuse rapidly across the peritoneum into the bloodstream, thus losing its osmotic potential over long periods of peritoneal exchange. To summarise, the semiquantitative data provided by the PET-2.27% would assist in providing an initial prescription, keeping in mind that HT patients would benefit more from APD and/or the use of icodextrin for long exchanges, but are clearly insufficient for exploring all possible causes of UF failure, since these results can only explain the component of this issue related to increased peritoneal permeability.
Other, more complex tests have also been described for measuring peritoneal diffusion with greater precision, including: peritoneal mass transfer coefficient as calculated using a complex mathematical model16 and the peritoneal permeability analysis described by Pannekeet in 1995,17 which measures small molecule transport using the mass transfer coefficient, in addition to estimating glucose absorption, serum protein clearance, and lymphatic reabsorption using a dextran 70 infusion. The biggest issue with using these tests in daily clinical practice is their high level of complexity and their inability to provide us with an adequate measure of the capacity and pathways used for water transport across the peritoneum.
The importance for determining patient prognosis that an adequately negative water and sodium balance in patients on PD has acquired18 has led the International Society for Peritoneal Dialysis (ISPD) to recommend measuring UF capacity with a 4-hour exchange using a hypertonic glucose solution.19 Peritoneal kinetic tests performed using 3.86%/4.25% glucose facilitate the measurement of free water transport (FWT) through aquaporins in their maximum condition of osmotic difference (using the sodium sieving measurement at 60 minutes) and provide for a standardised UF measurement.
In order to follow ISPD recommendations without losing the validity of historical reference values, several osmotic tests have been proposed based on the fundamental aspects of PET but with the incorporation of hypertonic glucose solutions. The mini-PET (1-hour exchange with 3.86% glucose) has been shown to be extremely effective at analysing FWT through aquaporines.20 The double mini-PET (two successive exchanges of 1 hour each with 3.86% and 1.36% glucose, respectively) combines this test with a measurement of osmotic conductance.21 The problem with these 1-hour tests is that the D/P creatinine results cannot be extrapolated to those obtained using a 4-hour PET.22 As such, the two tests would have to be performed separately: the double mini-PET on one day and the 4-hour PET-2.27% on another day, which implies a substantial increase in the duration of the examination process and the number of laboratory analyses needed.
Some authors have proposed combining the PET at 3.86% and the mini-PET into a single test, which would be feasible with a 4-hour exchange with hypertonic glucose together with a complete drain of the abdominal cavity at 60 minutes, at which point the sodium sieving and UF values would be measured. The fluid would then be re-infused and the test would continue for another 3 hours, allowing us to simultaneously measure FWT and small-pore UF, in addition to obtaining results for 4-hour D/P creatinine ratios.
Our initial proposal was to use a 4-hour PET-3.86% for several reasons: 1) this methodology is recommended by the ISPD in order to define UF failure (UF<400ml) and to obtain a standardised measure of UF25; 2) the sodium sieving value at 60 minutes allows us to examine aquaporin function; 3) the results obtained in terms of D/P creatinine ratios are comparable to those obtained using the PET-2.27%26 and also allow us to continue classifying patients based on their capacity for small molecule transport. However, based on the results from the La Paz group who used modified PET-3.86% with complete drainage at 1 hour, this test could be considered as a reference model for peritoneal kinetic analyses due to the fact that it retains all of the advantages of the PET-3.86% and is better at differentiating between small-pore UF and FWT.24
When should peritoneal kinetic studies be carried out in order to prescribe the modality of peritoneal dialysis during the monitoring period?
No clear consensus exists for this issue. Some guidelines recommend analysing peritoneal kinetics on a routine basis every 1-2 years,8,9 whereas others do not consider programmed peritoneal kinetics analyses to be necessary to maintain clinical stability (KDOQI10). What all guidelines do have in common is their recommendation that peritoneal kinetics can serve to help evaluate certain clinical problems such as metabolic disorders associated with malnutrition and insufficient dialysis, especially in the case of volume overload.
We do wish to point out here that peritoneal kinetic test are just one of many different tools used to examine these issues. For example, a patient with volume overload must first be evaluated for all possible causes of this phenomenon, such as: excess sodium intake, decreased residual diuresis, or drainage problems, etc., and after ruling out all of these other possibilities not related to peritoneal function, we would use the peritoneal kinetic analysis using hypertonic glucose solution. The kinetic analysis at 3.86% (with a complete drain at 1 hour, if possible) with a sodium sieving test at 60 minutes would inform us as to what type of transporter of small molecules the patient is, as well as aquaporin function, thus allowing us to optimise the treatment strategy, evaluate the true usefulness of icodextrin, and even indicate whether or not the patient should be transferred to haemodialysis (HD) if there is a UF failure associated with acquired HT with a loss of FWT.
Nor is there a consensus regarding whether peritoneal kinetics should be assessed after a case of peritonitis; this strategy is only recommended in the Australian guidelines.8 In several studies carried out by our research group, we have shown how episodes of peritonitis are a risk factor for developing HT and UF failure, both in the long-term4 and during the first few years of treatment.5 In a recent study assessing hypertonic kinetic analyses, patients that had suffered an episode of peritonitis had a lower sodium sieving value, which is an indicator for the later development of UF failure or increased transport rates.27 For this reason, we believe that assessing peritoneal kinetics one month after suffering an episode of peritonitis (even more so if the condition was aggressive) is recommendable, since this would aid in the early detection of patients at risk for UF failure.
To summarise this section in response to the question “is peritoneal kinetics useful for determining PD prescriptions?”, I would state:
Our recommendations for the use of peritoneal kinetics analyses for prescribing dialysis regimens are summarised in Table 1.
USEFULNESS OF PERITONEAL KINETIC ANALYSES FOR THE EARLY DETECTION OF DIALYSIS-INDUCED PATHOLOGICAL CHANGES
The use of the peritoneum as a dialysis membrane implies repeated exposure to non-biocompatible fluids (hypertonic glucose and GDP, lactate, acidic pH, etc.) and aggressions such as peritoneal infections, for which the membrane is not prepared. It is thus the obligation of the nephrologist to:
Do peritoneal kinetics analyses serve to examine the viability of the peritoneum and facilitate early detection of pathological changes induced by the dialysis technique that might limit its use?
The primary functional change that occurs over time on dialysis is the development of UF failure associated with acquired HT.3-5 After 5 years on dialysis, 20%-30% of patients develop UF failure, primarily due to increased permeability,4 but also due to anatomical changes such as the epithelial-mesenchymal transition (EMT).28 Both UF associated with acquired HT and EMT are recognised as pathologies that are induced by the dialysis technique itself, and as risk factors for the development of peritoneal sclerosis (PS).
Encapsulating PS (EPS) is a relatively uncommon but extremely severe complication that is clearly associated with time on PD (generally more than 5 years), although to date we have been unable to clearly identify which patients are at risk of developing it. A very low incidence of EPS (9 cases per 692 patients treated) was reported in the study carried out by Lambie et al.29 In their analysis of risk factors, they describe how two of these cases were associated with severe episodes of peritonitis before 5 years had passed, but the other 7 occurred without any clear history of peritonitis even after 5 years on PD. Upon review of the peritoneal kinetics analyses for these 7 patients in comparison with those from the rest of the at-risk population, the authors found that there were no differences in terms of initial water and solute transport characteristics, nor did they detect the development of small molecule HT until the diagnosis of EPS and suspension of PD. The authors do describe a loss in UF capacity starting 2 years after the clinical diagnosis, and this decrease was detected through a PET-2.27%. It is possible that if kinetic analyses had been performed using 3.86% glucose and sodium sieving, patients at risk for developing UF failure and EPS would have been detected earlier and with greater precision. In a study by La Milia et al.30 involving 95 patients who underwent a baseline PET-3.86% and were prospectively monitored using a peritoneal kinetic analysis (PET-3.86%) every year for five years, the Cox analysis revealed a decrease in 60-minute sodium sieving values as the only factor associated with UF failure. Some histopathological studies have suggested that a loss of function of aquaporins could be associated with angiopathy and fibrosis.31
Those authors who argue against the use of peritoneal kinetic analyses routinely state that not all UF failures result in EPS, and that while UF failure is a relatively common occurrence (as much as 50% of patients with more than 5 years on PD), EPS is a very rare condition. For our part, we who defend the routine use of peritoneal kinetic analyses base our argument on the fact that EPS is an extremely severe disease associated with a very high mortality rate, and once the clinical symptoms appear, there are very few options available for treatment, making detection in early stages an essential therapeutic strategy, since certain measures such as peritoneal rest can at least partially revert this process if applied early enough.22 In patients detected as high risk, transfer to HD along with the use of tamoxifen appears to improve the prognosis of EPS,33 and it has been well established that if the sclerosing process has already started, removal from the dialysis technique is not sufficient for halting its progression.
To summarise:
- Close monitoring of permeability and UF capacity through peritoneal kinetic analyses using hypertonic glucose solution.
- Evaluation of treatment using peritoneal rest periods with heparin.
- In the case of persistent UF failure, the patient must be definitively transferred to HD and evaluated for the possibility of treatment with tamoxifen.
Does the patient’s initial transport type influence survival on peritoneal dialysis?
In the last 15 years, an important debate has evolved regarding whether patients who are initially high transporters have worse global survival or survival on dialysis. Studies from the late 1990s and early 2000s, focused primarily on patients on CAPD, certainly showed this trend,34-37 although this finding was not confirmed in all cases.38,39 As mentioned in the previous section, the meta-analysis carried out by Brimble et al,12 which was based primarily on the four first studies and the results from a New Zealand registry,40 appeared to confirm the inferior prognosis on PD of patients who initially are high transporters, and the recommendation was even made that these patients should be directly transferred to HD.41
Our experience42 and that of other authors, based on the analysis of retrospective data, does no confirm this inferior prognosis if all of the currently available tools are used, such as APD and icodextrin.13,14 The beneficial effects of icodextrin for delaying the development of acquired HT in prevalent anuric patients were shown in a sub-analysis of the EAPOS study.43 The possibility does exist that, if the clinician does not perform peritoneal kinetic analyses, dialysis prescriptions will not be adapted early to each patient’s particular transport characteristics, which can worsen the prognosis. In this context, our group analysed the prognostic importance of baseline transport characteristics, finding that, during the first year, the global sample of patients experienced a non-significant decrease in permeability and an increase in UF capacity. This phenomenon was most evident in patients with initial UF failure or HT44 in which the change was significant, such that the issue was at least partially corrected in a high percentage of these individuals. In a more recent study, we confirmed this phenomenon, but also observed that the decrease in permeability and increase in UF described in the global sample did not occur5 if the patient is exposed to high glucose concentrations during the first year. This led us to carry out another study to analyse what would happen if patients were treated from the start with icodextrin solutions: in this case, the decrease in permeability and increase in UF during the first year reached statistical significance, with a much greater quantitative difference than those described using conventional dialysate solutions.45
Do peritoneal kinetic analyses serve to evaluate possible improvements in clinical results from using more biocompatible dialysate solutions?
In recent years, new PD solutions have been developed with the intent of improving biocompatibility and avoiding aggressions to the peritoneum. These new dialysate solutions are theoretically superior, since they reduce the quantity of GDP and lactate present, along with providing a more physiological pH. Several studies carried out both in animal models and in patient biopsies have shown the benefits of these solutions.
However, the most difficult task remains before us: to demonstrate in clinical practice whether the repeated use of these solutions provides any advantages in terms of functional and anatomical protection of the peritoneum. In our case, after performing peritoneal kinetic analyses systematically using conventional solutions and continuing to perform them using the new solutions available, we are at the stage in which we can compare and analyse the early advantages of these new solutions. One example is the previously mentioned study in which we analysed the effect of the use of icodextrin starting as the initial treatment strategy45: since we had access to a database of information regarding the state of each patient’s peritoneum during the first year with conventional solutions, we were quickly able to compare results and confirm that the decrease in permeability and increase in UF were greater with icodextrin solutions, thus providing a protective effect on the peritoneum.
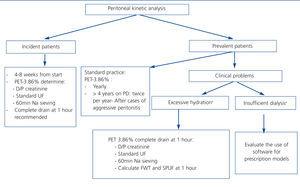
Our recommendations for the use of peritoneal kinetics analyses for the early detection of pathological changes induced by peritoneal dialysis are summarised in Table 2. In Figure 1, we make a general proposal for the use of peritoneal kinetics in daily clinical practice.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Recommendations for the use of peritoneal kinetic analysis for determining dialysis prescriptions
Table 2. Recommendations for the use of peritoneal kinetic analysis for the detection of pathological changes induced by peritoneal dialysis
Figure 1. Schematic of the global recommendations for the use of peritoneal kinetics analysis in clinical practice