La mitad de los enfermos con síndrome nefrótico causado por glomeruloesclerosis focal y segmentaria (GFS) primaria presentan resistencia al tratamiento con esteroides. En caso de corticorresistencia, la mejor opción basada en la evidencia ha sido clásicamente el tratamiento con inhibidores de calcineurina, aunque estudios recientes indican que micofenolato podría tener una eficacia similar. En los enfermos con resistencia a anticalcineurínicos, no existe ninguna opción capaz de modificar el curso clínico de la enfermedad, avalada por ensayos clínicos de diseño apropiado, aunque en estudios observacionales se ha sugerido la posible utilidad de micofenolato, sirolimus, rituximab, aféresis o altas dosis de galactosa como opciones terapéuticas. En las GFS de origen idiopático, resistentes a esteroides y anticalcineurínicos, antes de tomar la decisión de ensayar o no otros fármacos inmunosupresores, podría ser apropiado realizar un análisis sistemático que contemplara: 1) considerar si la dosis y el tiempo de tratamiento con esteroides y anticalcineurínicos fueron adecuados; 2) analizar el nivel de expresión de la glicoproteína P en los linfocitos; 3) considerar realizar una nueva biopsia renal en caso de que en la primera no se disponga de estudio de microscopía electrónica; 4) en enfermos jóvenes, considerar un estudio genético para descartar la presencia de la variante p.R229Q de la podocina en combinación con mutaciones heterozigotas en NPHS2, y 4) considerar la gravedad y dificultad de manejo del síndrome nefrótico y la probabilidad de pérdida progresiva de la función renal. En la actualidad, hay múltiples vías de estudio para intentar identificar los mecanismos patogénicos causantes de la lesión podocitaria y hay también en curso varios estudios para analizar la eficacia de fármacos como adalimumab, fresolimumab, rosiglitazona, ACTH (corticotropina) o galactosa a altas dosis, cuyos resultados preliminares han generado expectativas que requieren ser confirmadas en estudios clínicos a mayor escala. En un futuro, es posible que el mejor conocimiento de la vía o vías patogénicas causantes de GFS permita diferenciar entre las formas inmunomodulables y las que no lo son, pero, hoy por hoy, este desafío continúa plenamente vigente.
Half of patients with nephrotic syndrome caused by primary focal segmental glomerulosclerosis (FSGS) have resistance to treatment with steroids. In the case of corticosteroid resistance, the best evidence-based option has classically been treatment with calcineurin inhibitors, although recent studies indicate that mycophenolate may have similar efficacy. In patients with resistance to calcineurin inhibitors, there is no option that allows the clinical course of the disease to be modified, and this is supported by appropriately designed clinical trials, although observational studies have suggested the potential usefulness of mycophenolate, sirolimus, rituximab, apheresis or high galactose doses as treatment options. In FSGS of idiopathic origin, resistant to steroids and calcineurin inhibitors, before taking the decision whether or not to test other immunosuppressive drugs, it might be appropriate to conduct a systematic analysis that considers: 1) evaluating whether the dose and duration of treatment with steroids and calcineurin inhibitors were suitable, 2) analysing the level of P-glycoprotein expression in lymphocytes, 3) performing a new renal biopsy if there is no electron microscopic study available for the first, 4) in young patients, considering a genetic study to rule out the presence of the podocin variant pR229Q in combination with heterozygous mutations in NPHS2, and 5) evaluating the seriousness and difficulty of managing the nephrotic syndrome and the likelihood of progressive loss of renal function. Currently, there are multiple study avenues that attempt to identify the pathogenic mechanisms that cause podocyte injury and there are also several studies underway to analyse the efficacy of drugs such as adalimumab, fresolimumab, rosiglitazone, ACTH (corticotropin) or galactose at high doses, whose preliminary results have generated expectations that require confirmation in larger-scale clinical studies. In the future, it is possible that a better understanding of the pathogenic pathway or pathways that cause FSGS may allow differentiation between immunomodulable and non-immunomodulable forms, however, this continues to be a challenge currently.
INTRODUCTION
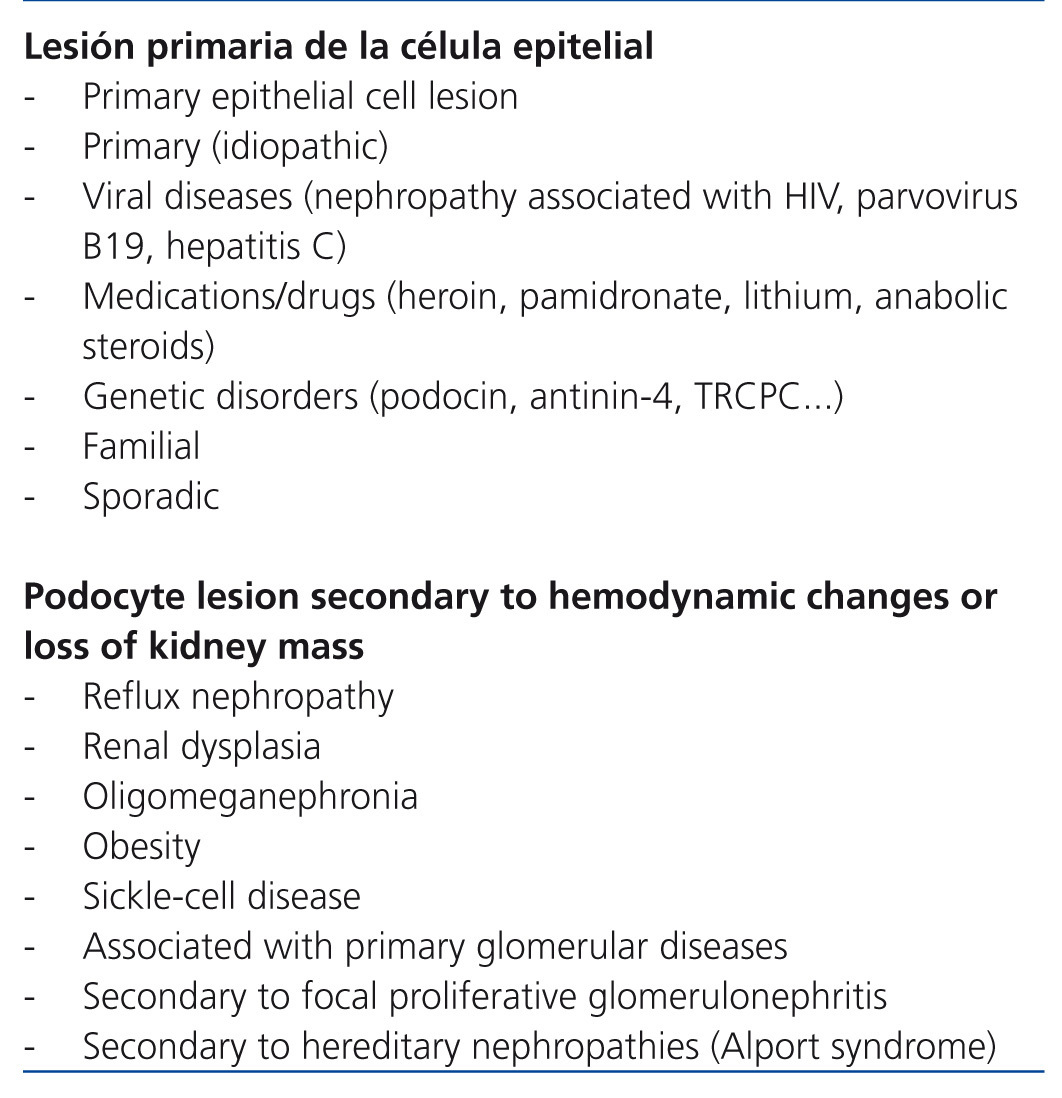
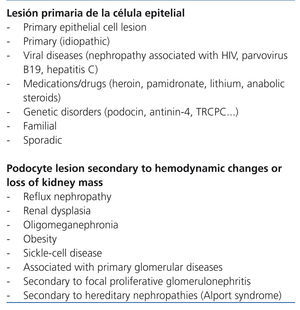
The term focal segmental glomerulosclerosis (FSGS) defines a clinical entity that has a characteristic lesion pattern on optic microscopy but with multiple possible aetiologies.1 In practical terms, FSGS is classified as primary or secondary depending on whether the aetiology is identified or not.1,2 Nevertheless, as the criteria used in different classification systems to refer to primary and secondary forms are not the same, a detailed definition of the term primary FSGS is needed. In some classifications (Table 1), the term is reserved for idiopathic cases, excluding forms that are due to mutations in podocyte proteins and those due to viral infections or drug toxicity. Conversely, in some studies (Table 2) the concept of primary form is identified by the primary podocyte lesion regardless of whether or not it is of unknown, viral, genetic or pharmacological aetiology. In terms of indications for immunosuppressant treatment, the classification shown in Table 1 is useful since the use of these drugs is difficult to justify in patients with evidence of podocyte mutations and, in cases in which viral infection or pharmacological toxicity is shown, the more proper focus is treating the infection or suppression of the causal agent. Nevertheless, even accepting cases that are truly idiopathic as primary, it cannot be ruled out that some patients selected for immunosuppression treatment have forms that, as understanding of the pathogenesis of FSGS progresses, will someday be classified as secondary or not sensitive to immunosuppressants. With these exceptions, and despite doubts about whether or not there is a single common pathogenic pathway that is immune-modifiable for all forms of idiopathic FSGS, currently available information3-6 agrees that in patients with idiopathic forms of FSGS that feature persistent nephrotic syndrome, steroids are the first therapy choice and in the absence of a response to them, the best option is treatment with calcineurin inhibitors.4-6 However, approximately 40% of patients are resistant after initial treatment with these drugs or develop resistance after one or several recurrences. Resistance to treatment is a difficult problem and has severe consequences since it is one of the most important independent predictors of developing progressive chronic kidney disease.7-10
This review explains the consensus rules and the outcomes that can be expected after steroid and calcineurin inhibitors therapy in idiopathic FSGS. The available therapeutic options are considered for patients who are resistant to these drugs. In addition, the clinical variables that may provide guidance when deciding between symptomatic treatment or prescribing immunosuppressant treatment are considered. Finally, an analysis is made of where current research is heading.
DIMENSION OF THE PROBLEM. GUIDELINES AND THE RESULTS OF TREATMENT WITH CORTICOSTEROIDS AND CALCINEURIN INHIBITORS IN FOCAL SEGMENTAL GLOMERULOSCLEROSIS
Corticosteroids
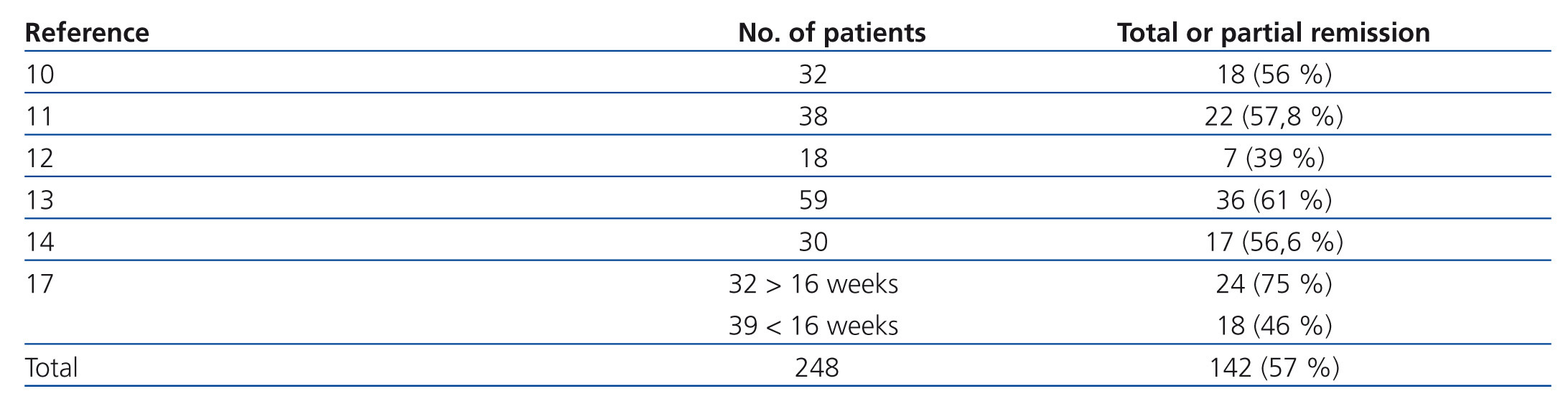
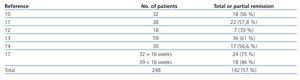
The probability of a response to steroids depends on initial renal function, the level of podocyte loss, time of disease progression and extension of interstitial fibrosis lesions.7-9 Recommending steroid treatment is based on the results of observational studies in case series (Table 3).10-17 The duration of treatment and the steroid dosage is based on two aspects without high levels of evidence; because of this, it is very difficult to define clear and undeniable criteria. For regimens lasting less than 12 weeks, the percentages of remission are no greater than 30% while the highest frequency of remission has been described with treatments from 6 to 12 months.2-6,17 It is recommended that treatment be started at a 1mg/kg/day dosage and monthly monitoring of urine protein excretion be performed. Given that exposure to such high steroid doses for long periods of time can lead to serious toxic effects, and that the majority of patients who will respond show a certain reduction in proteinuria within 14 to 16 weeks, the steroid-resistance criteria can be accepted if there has not been any change in urine protein excretion within 14-16 weeks or if there has been, the patient persists with nephrotic syndrome after having finished a 6-month treatment cycle. With this criteria, remissions have been described in 60%-70% of patients with normal renal function at the baseline evaluation.4-6,17 About 25% of patients maintain stable remission following steroid treatment and approximately 50% have steroid resistance after the initial treatment or develop it after one or several recurrences.2,10-17
Calcineurin inhibitors
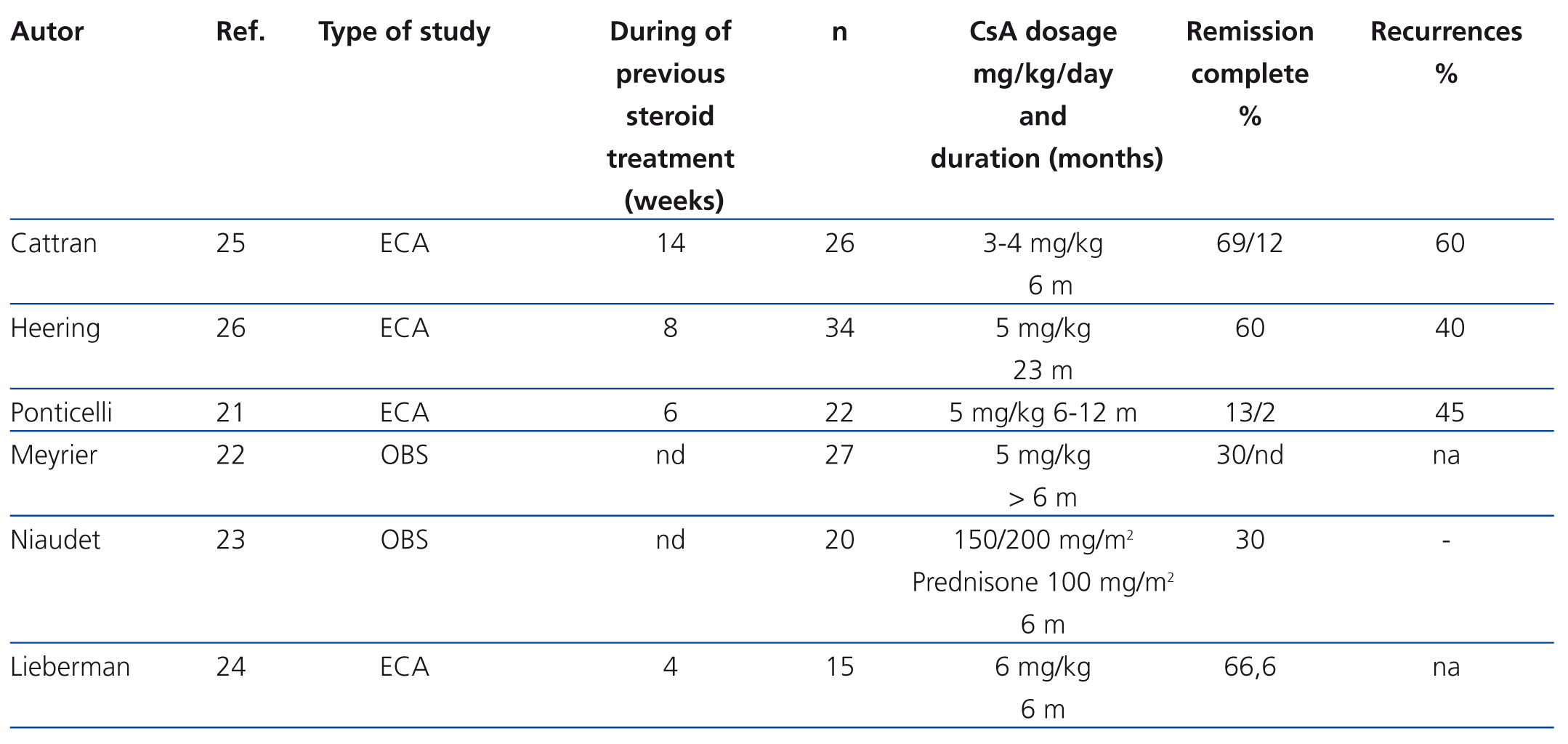
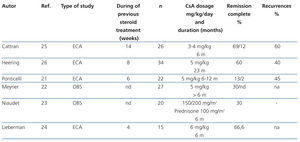
The indication for calcineurin inhibitors in the treatment of FSGS is based on the hypothesis that the podocyte lesion results from the activation of the immune response.18,19 However, it has been demonstrated that these drugs also have direct effects on intracellular signalling pathways and the architecture of the podocyte cytoskeleton.20 The efficacy of cyclosporine A (CsA) for the treatment of steroid resistance has been described in several observational studies and is backed by the results of randomised clinical trials (Table 4).21-26 It is recommended that treatment be started at a dosage of 5 mg/kg/day, divided into two doses, administered every 12 hours, in order to maintain trough values between 150ng/ml and 200ng/ml. Treatment should be maintained for a minimum of 6 months and, if remission of nephrotic syndrome has not been achieved at the end of this period, the patient should be considered to be resistant to CsA. Complete or partial remission is usually induced with this regimen in 60%-70% of the cases.2-7 In case of remission, it is recommended that treatment be continued for a minimum of 12 months and that it then be reduced gradually by 25% every 2-3 months until suppression or evidence of a recurrence is detected. Longer treatment is recommended in recurrent cases. However, there is no evidence that allows for the recommendation of a specific period of treatment.2,3,5,6 15-20% of cases develop dependence and require continuous treatment with low dosages of CsA in order to maintain remission. About 40% of steroid-resistant patients are also initially resistant to CsA. Half of cases that respond become resistant to CsA. The data on tacrolimus in adults with steroid-resistant FSGS are limited to observational studies27-30 that suggest that this drug has a similar efficacy and toxicity profile to CsA. A clinical trial on children with steroid-resistant FSGS31 has been published in which the efficacy of CsA is compared with tacrolimus, in both cases with concomitant use of low-dose steroids, and it was concluded that both drugs have similar efficacy with different extrarenal adverse effect profiles and a tendency towards a lower number of exacerbations with tacrolimus. Based on these criteria, tacrolimus may be an option instead of CsA in some patients. In adults, it is recommended that treatment be initiated at a dose of 0.06mg/kg and then adjusted to maintain trough values between 7ng/ml y 9ng/ml since higher dosages (0.15 mg/kg) are associated with a high incidence of nephrotoxicity.28 In children,31 somewhat higher dosages have been used (0.1mg/kg-0.2mg/kg) with no apparent risk of nephrotoxicity. There are no data on the duration of treatment nor the steps to take if total or partial remission of proteinuria is obtained. However, the recommendations made for treatment with CsA are probably also valid for treatment with tacrolimus.
The use of calcineurin inhibitors should be considered to be contraindicated in patients with altered renal function. It seems reasonable to perform very frequent follow-up (monthly) of renal function in patients with even mild reductions in glomerular filtration (<80ml/min) and not to recommend use in cases in which the glomerular filtration levels are less than 60ml/min.2-5
While it is usually advisable to wait until the patient meets well-defined criteria for steroid resistance before prescribing calcineurin inhibitors, in cases in which steroid treatment is associated with relevant adverse effects or in cases in which they are poorly tolerated, it is advisable to decrease the exposure period to these drugs and/or reduce the dosage and to add calcineurin inhibitors early. If a response is not obtained after 6 months of treatment with these drugs, with or without concomitant low-dose steroids, the patient should also be considered resistant to these drugs. In this situation, which occurs in 45%-50% of adult patients with FSGS, there is not treatment so far for which the efficacy has been compared in randomised clinical trials that has been capable of modifying the clinical course of the disease.
TREATMENT OPTIONS FOR PATIENTS WITH STEROID AND CALCINEURIN INHIBITOR RESISTANCE
Mycophenolate
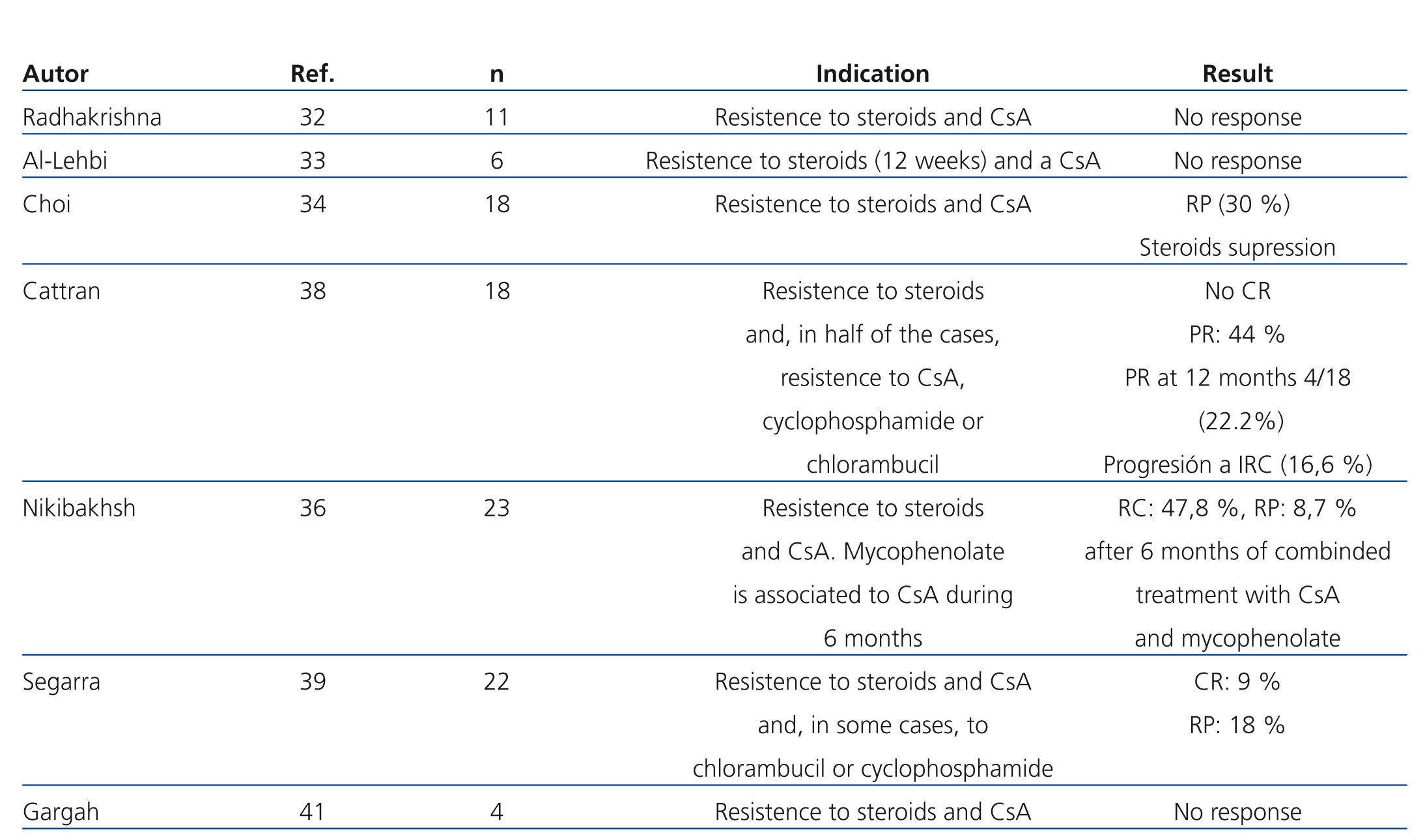
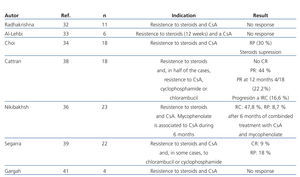
The efficacy of mycophenolate (MMF) in steroid and calcineurin inhibitor resistant patients has not been compared in high-quality studies. In several observational studies in which patients with different criteria for steroid resistance and those treated with MMF at different dosages and for various lengths of time were included, a significant reduction in proteinuria has been described in a percentage of patients that varies between 30% and 50%. However, there was a very small number of total or partial remissions (Table 5).32-41 In a recent multicentre clinical trial,42 the efficacy of concomitant MMF and dexamethasone (Dx) versus CsA was analysed in patients with steroid-resistant FSGS, with no differences found between both groups in the short or long term. The percentage of remission achieved in both arms was similar (30% in the MMF + Dx group versus 45% in the CsA group) and lower than that observed in previous clinical trials using CsA versus placebo (60%-70%). The study suggests that MMF could be an alternative to CsA in the case of steroid resistance. Nevertheless, it suffers from design flaws that make analysis of the results difficult. Because it included patients with non-nephrotic proteinuria, secondary forms were not adequately ruled out and the concept of steroid resistance is defined using a different criteria than that used in the majority of guidelines. For these reasons, the results obtained cannot be extrapolated to those patients with primary forms who suffer from nephrotic syndrome that meet formal criteria for steroid resistance.
mTOR inhibitors
Studies on sirolimus in patients with steroid-resistant FSGS are very scarce. Two observational studies43,44 have described favourable results with significant reductions in proteinuria and even total or partial remissions of nephrotic syndrome. A phase II trial, designed to evaluate the safety and efficacy of sirolimus in patients with FSGS that is resistant to other immunosuppressants, including steroids, had to be halted prematurely due to evidence of an increase in proteinuria and deteriorating renal function in 5 of the 6 patients included.45
Alkylating and cytotoxic drugs
Observational studies in steroid-resistant patients describe a probability of response of 25%, clearly lower than that seen with calcineurin inhibitors.24,46,47 Therefore, these drugs are not considered to be a good treatment option in cases of steroid resistance. Their only possible indication, though disputable since it is not backed by any evidence, would be the treatment of patients with nephrotic syndrome in which treatment with calcineurin inhibitors or MMF would not be advisable due to the risk of renal or extra-renal toxicity.
Rituximab
Isolated cases and observational studies that include few patients48-51 on the efficacy of treatment with rituximab have been reported in patients with steroid-resistant FSGS. The results conflict on both effect on proteinuria as well as on renal function and the studies are heterogeneous on their definition of resistance to prior immunosuppressant treatment. A reasonable recommendation on the whether or not rituximab should be indicated in patients with steroid-resistant FSGS cannot be made with the currently available evidence. However, the limited data on adult patients do not suggest a high expectation of success.51,52 It may seem paradoxical that even though the results are also discordant, treatment with rituximab, which is often used in conjunction with plasmapheresis, has been associated with a higher treatment success in recurrent post-transplant FSGS53-61 than native kidney FSGS. However, the data observed in post-transplant recurrence cannot be extrapolated to primary FSGS of a native kidney for a variety of reasons. First, given that the percentage of patients with FSGS who suffer post-transplant recurrence is around 30%,59 it is highly probable that the recurrence is associated with a specific pathogenic mechanism that is not the same as that which causes non-recurrent forms. Second, the recurrence in transplant patients occurs in a setting of intense and combined immunosuppression, so the mechanisms that are responsible for this appear not to be very sensitive to immunomodulation. Finally, post-transplant recurrence is usually diagnosed by evidence of recurrence of proteinuria that in some cases can even not reach the nephrotic range.61 Therefore, it is very possible that treatment in recurrent forms is initiated in less advanced phases of the podocyte lesion and, as a result, have a greater probability of response to treatment. The use of rituximab in the treatment of steroid-resistant FSGS is based on the hypothesis that the podocyte lesion is caused by an immune-modifiable pathogenic mechanism. However, recent evidence indicates that rituximab may reduce proteinuria by acting directly on the podocyte.62
Apheresis
The basis for the use of these techniques in the treatment of FSGS is based on the hypothesis that the presence of a circulating factor is capable of altering the patency of the filter membrane and causing irreversible damage to the podocyte.19 The hypothesis was developed from evidence of recurrences following kidney transplant53-61 and from evidence of transmission of nephrotic syndrome from mothers with FSGS to newborns.63 The results described with plasmapheresis and immunoadsorption in patients with steroid-resistant FSGS in a native kidney are based on small case series in which the criteria for steroid resistance and the treatment guidelines used differed significantly.64-68 Favourable results were described in some studies in patients with multiple drug resistance while in others not favourable effect was seen. An in vitro test has been described for demonstrating the presence of the supposed circulating factor and the response to immunoadsorption has been associated with the presence of this factor,19,69,70 but these data lack external validation. More recently, a soluble urokinase receptor has been identified as one of the possible circulating mediators for podocyte injury and it has been described that, in recurrent FSGS in the transplanted kidney, plasmapheresis reduces levels of this factor and may induce remission.71 These data have not been demonstrated in FSGS in a native kidney. Favourable results have also been described using selective LDL-apheresis techniques72-74 but the pathogenic basis to justify its beneficial effect is unknown and clinical experience is very limited.
Combination immunosuppressant treatments
No treatment has been described in adults with demonstrated steroid and calcineurin inhibitor resistance that can modify the clinical course of the disease. In a recent observational study,75 the efficacy and safety of combination of CsA and MMF for 12 months was evaluated in 27 adult patients with FSGS and well-defined criteria for steroid and CsA resistance. The treatment did not induce remissions of nephrotic syndrome in any cases nor did it modify the glomerular filtration slope. The results appear to be more optimistic for paediatric patients since the combination of CsA and MMF in patients with previous steroid and CsA resistance has been described as being able to induce remission in more than 50% of cases (complete 48%, partial 8.7%).36
WHAT IS THE PROPER ATTITUDE IN THE CASE OF STEROID AND CALCINEURIN INHIBITOR RESISTANCE?
The use of the drugs and techniques mentioned above have in common a low level of evidence to support their efficacy, an absence of reliable indicators for predicting the outcome and a low but not absent probability of response. This last point is very important since it raises the expectations that a specifically identified patient may benefit from them. This scenario, in which decision-making is not easy and doubts are the norm, puts the professional in the dilemma of choosing between prescribing conservative treatment in order to control the complications associated with nephrotic syndrome, thereby minimising exposure to possible adverse effects from immunosuppressants, or continuing a chain of immunosuppression, with its uncertain results and possible long-term risks, with the confidence that one of the prescribed treatments will induce remission of the process. In the case of prescribing the second option, doubts are often raised about when to stop trying new drugs when there has been no response to previous ones. Clinical guidelines3,6 often provide information on the available evidence and leave the use or avoidance of these drugs up to the physician's judgment. This indicates that the problem is currently far from being resolved. Nevertheless, there are some considerations, though they have significant limitations, which may be considered when making decisions.
Analysing the possible causes and the type of steroid resistance
Though it surely appears obvious, detailed review of the treatment regimen is very useful in some cases for ensuring that the patient meets the formal criteria for resistance and that this, in reality, is not due to underexposure to the drug. Once this possibility has been evaluated, the reasons why some patients respond to steroids and others do not are as unknown as the pathogenesis of FSGS. It is logical to assume that that absence of a response to steroids may be directly related to the pathogenesis or to a certain threshold of irreversible podocyte injury. However, there is also evidence indicating that there may be pharmacodynamic reasons associated with the level of cellular glycoprotein P expression.76 Glycoprotein P is a transmembrane protein that acts as a transporter responsible for cellular efflux of drugs and toxins with a molecular weight between 300 and 2000 Da, among which are included xenobiotics or drugs such as vinca alkaloids, verapamil or corticosteroids, among others.77-78 Overexpression of glycoprotein P is considered to be one of the mechanisms that cause resistance to chemotherapy in oncology patients79 and in steroid resistance in autoimmune diseases such as systemic lupus erythematosus or rheumatoid arthritis.80-82 Recently, it has been shown that interleukin (IL) 2 may induce an increase in glycoprotein P expression in lymphocytes.83-85 Through this mechanism, lymphocyte activation may contribute not only to the pathogenesis of nephrotic syndrome, but also to the development of steroid resistance, especially in patients who have been repeatedly exposed to these drugs over long periods of time.76 These data are of great potential interest in that they may be useful for predicting the response to treatment with steroid, the risk of subsequent recurrence or steroid resistance and/or for recommending early introduction of calcineurin inhibitors based on the levels of expression and functionality of glycoprotein P at the time of diagnosis or over the course of the disease during follow-up. The initial response to steroid treatment may orient one towards the type of resistance to some measure. In patients who develop steroid resistance after one or several recurrences after a good initial response, it may make more sense to investigate pharmacodynamic causes of resistance than in patients who have never responded to steroids.
Reanalysing the diagnosis: Considerations on rebiopsy and genetic studies
While it is important to try to ensure that the patient has idiopathic FSGS prior to starting immunosuppressant therapy, when this fails to induce remission it seems reasonable and prudent to bring up the need to reanalyse the available clinical and histological data in detail (to the measure that currently available resources allow) in order to ensure that one is dealing with a primary form of FSGS and to decide whether or not a study should be done to rule out sporadic genetic forms, starting with the foundation that the probability of this being the case is very low if the disease onset was during adulthood and in the absence of a family history. Differentiating between primary and secondary forms of FSGS is based on the clinical profile and the ultrastructural renal examination using electron microscopy.1,2 Although this criterion is not unquestionable, idiopathic forms are generally characterised by nephrotic syndrome and generalised obliteration of podocyte feet on electron microscopy. In forms which are secondary to reduction of renal parenchyma, hyperfiltration and obesity, proteinuria in the nephrotic range can be observed, but the presence of nephrotic syndrome is unusual and the electron microscope examination reveals that the obliteration of podocyte feet has a more diffuse focal segmental distribution.86 Despite the fact that this differentiation between extreme cases appears to be clear, when electron microscopy studies are not available the differentiation can be difficult. Additionally, secondary forms caused by viral infections, drug toxicity or podocyte mutations can have a clinical and microscopic profile that is indistinguishable from the idiopathic forms and, therefore, are only identifiable if active investigation is done, either systematically or based on the clinical context in which the nephrotic syndrome is present.
In patients in which the initial biopsy revealed unequivocal signs of FSGS with a well-defined pattern on optical and electron microscopy, the usefulness of a rebiopsy in the case of resistance is questionable and possibly only justifiable for evaluating the progression of interstitial fibrosis. Performing a second biopsy may be useful in cases that show primary or secondary resistance, those in which the lesions seen on the first biopsy were poorly expressive (synechiae, suspected cellular variant) and if an electron microscope study is not available that would allow for evaluation of the status of podocytes in the glomerulus when the appearance is normal on optical microscopy. In these cases, the rebiopsy should include an electron microscopy study and allow for evaluation of whether or not the lesions have progressed towards a better defined pattern.
With regards to genetic study, the indication and the possible mutations to be studied depend on the age at onset, the presence or absence of associated extrarenal syndromes, a family history and, in this last case, the observed pattern of inheritance. It is often recommended that a genetic study be performed in all congenital cases in children with steroid-resistant forms. In young adults with steroid resistance, the frequency of mutations in the literature is very low. There is no agreement on the type of genetic study that should be done and systematic investigation of mutations is usually not recommended. However, some proposals have recently been made based on data from epidemiological studies.87,88 In a study in which patients were included from different ethnicities and geographical origins,87 up to 10% of patients of European or South American origin with a Hispanic background whose disease onset was during adulthood or young adulthood (average age 19 years) may present a pathogenic heterozygotic mutation associated with the podocin p.R229Q variant. However, these data have not been confirmed in other studies that included patients from other geographic locations in which the coinciding p.R229Q with mutations at NPHS2 seen was significantly lower (0%-2%).88-90 Other authors91 have described mutations at NPHS2 or TRCP6 in 43% of patients with familial forms and in 4 of 41 cases (14%) (1 NPHS1, 2 NPHS2 and 1 TRCP6), with sporadic forms of steroid-resistant nephrotic syndrome with an age at onset older than 18 years. This evidence, though very limited, should not be ignored before submitting the patient to the prolonged effect of immunosuppressant treatment. Therefore, without more data, a reasonable approximation for the sporadic cases that have an onset prior to the third decade of life, which have demonstrated resistance to both steroids and calcineurin inhibitors, may be to perform a genetic study to identify the presence of variant pR229Q which has been associated with pathogenic mutations at NPHS2.92 Evidence of the presence of this association has clear practical implications since it is a useful argument for not prescribing immunosuppression treatment,93 allows for advising patients if they plan to have children and, if a kidney transplant is proposed, it is useful for selecting possible family donors.
Adequate risk snalysis. The importance of prognosis variables
While it is true that steroid resistance has been identified as the primary factor for a poor prognosis since it involves higher risk of progressive deterioration or renal function,9,10 this does not mean that all patients with steroid resistance will definitely progress to advanced stages of chronic kidney disease. A variable though small percentage of patients, after prolonged follow-up, maintain unaltered renal function despite the persistence of nephrotic syndrome. Therefore, the risk of increasing exposure to immunosuppressants should be weighted according to the clinical progress expectations. For this, there are two very important aspects: 1) analyse the type and extension of the lesion seen on kidney biopsy in detail and 2) have a minimal follow-up in order to determine whether the absence of a response implies a risk of progression towards chronic kidney disease or if it is limited to the risk of complications associated with nephrotic syndrome. In the case of the latter, it would be recommended that one consider if the nephrotic syndrome requires simple or complex management based on the source of difficulty for controlling oedema and what are the consequences of persistent proteinuria (malnutrition, dyslipidaemia, infections, episodes of thrombosis, need for long-term anticoagulant therapy). For lesions observed on kidney biopsy, it is undeniable that the presence of extensive interstitial fibrosis and advanced segmental sclerotic lesions imply a poor prognosis and reduce the probability of a response to immunosuppressants. Date on the prognostic value of different FSGS variants described on optical microscopy1,7 indicate that the collapsing forms (whose addition to the category of FSGS is a source of controversy) have a poorer prognosis than other variants and that perihilar forms are more often associated with secondary/adaptive FSGS. Although the tip lesion variant has been described to be associated with a greater probability of response to corticosteroids and a better prognosis, the prognostic differences between tip lesions, classic forms (NOS) and cellular forms have not been adequately validated and, therefore, the histological variant is currently considered of little usefulness for making decisions about treatment.
FUTURE PERSPECTIVES. HOW FAR WILL WE GO?
The currently available evidence of scant response that is obtained from patients who are resistant to immunosuppressant therapy has led researchers down two parallel lines of investigation: 1) the study of new drugs that are capable of modulating different aspects of the inflammatory response or the fibrosis process and 2) the study of new pathogenic and/or biomarker pathways that allow for identification of patients based on what their response to treatment will be.
New treatments in the research phase
In this aspect, studies carried out with adalimumab (a monoclonal antibody that targets tumour necrosis factor α (TNFα)), fresolimumab, a monoclonal antibody that targets transforming growth factor beta (TGFβ), rosiglitazone, ACTH and high-dose galactose are notable.
Preliminary results of phase I studies have been reported in which the efficacy of adalimumab and rosiglitazone in steroid-resistant FSGS are analysed. The results after 16 weeks of treatment indicate that approximately 50% of patients treated with adalimumab and 71% of patients treated with rosiglitazone may stabilise renal function and show a reduction in proteinuria. Both drugs are being evaluated for efficacy and safety in phase III studies.94-96 A study is also in the development stage to analyse the potential efficacy of fresolimumab97 and ACTH.98 The idea of analysing the efficacy of high-dose galactose is based on the evidence that one of the recently identified soluble factors that may be a possible mediator of podocyte injury (cardiotrophin-like cytokine factor 1) has a high affinity for galactose.99 Positive results have been reported so far on galactose treatment in recurrent FSGS following kidney transplantation.100,101 However, the results described are difficult to attribute to galactose due to the fact that the patients received other concomitant treatments including plasmapheresis. Remission of nephrotic syndrome has also been described following galactose treatment in native kidney FSGS.102
Biomarkers that are predictive of treatment response
No clinical, histological or biochemical marker has been identified so far that would allow for differentiation of patients based on their response to steroids or calcineurin inhibitors. On a histological level, increased podocyte expression of CD80103,104 and reduction of alpha-dystroglycan expression105-106 has been shown to allow for differentiating nephropathy through minimal changes in FSGS. Therefore, in cases of doubtful lesion on optical microscopy, this may be useful in predicting the response given the greater corticosteroid sensitivity in the first of the two entities. Urine CD8099 and TGFβ107 levels have also been proposed as candidates for differentiating between both processes. However, its association with treatment response still has not been analysed. In addition to the association between ABCB1/glycoprotein P expression and steroid resistance,76,81 a possible association between certain polymorphisms in genes that code for IL-6, IL-4 and TNFα and the response to steroids in children with idiopathic nephrotic syndrome has been described.108 Urine proteomic profiles that would be different based on the response to steroids have also been described, but these have still not been evaluated in clinical studies.109-111 Finally, circulating levels of urokinase soluble receptor (suPAR) have very recently been reported as being elevated in patients with primary FSGS but not in patients with other glomerular diseases.71 Analysis of the association between suPAR levels and the patients’ clinical characteristics, response to treatment or prognosis are still based on very limited data that indicate that the association may be complex. In a recent study,112 suPAR levels were measured in two patient cohorts. The first cohort included patients recruited in the clinical trial carried out in the US to analyse the efficacy of combining MMF and Dx versus CsA in patients with steroid resistant FSGS.42 The second cohort included patients under 18 years of age recruited in the European PodoNet consortium for the study of steroid-resistant nephrotic syndrome. In both cohorts, it was noted that baseline suPAR levels were significantly higher in the healthy controls, but with a heterogeneous distribution of levels. In the first cohort, it was confirmed that 84.3% of patients had baseline suPAR levels that were greater than the cutoff point of 3000pg/ml (selected by the same authors as the previous study71 as optimal for differentiating patients with primary FSGS from other forms of nephrotic syndrome). Baseline suPAR levels were associated with glomerular filtration rate and were greater in black-race patients. After completing 26 weeks of treatment, despite not having any differences in the number of remissions between both groups, the suPAR levels increased in patients treated with CsA and decreased in those treated with MMF, with significant differences between both groups in the multivariate analysis after adjusting for possible confusion factors. The suPAR levels were independent from proteinuria and C-reactive protein both before and after treatment. Baseline suPAR levels, glomerular filtration and treatment with MMF were the only independent predictors of absolute changes in suPAR levels after treatment, but neither baseline suPAR levels nor progress following treatment were predictors of the outcome. On the contrary, relative changes in suPAR levels were independent predictors of the probability of obtaining complete remission after treatment, after adjusting for age, sex, ethnicity, glomerular filtration and baseline suPAR levels. In patients who went into remission, the increase in suPAR levels after complete remission was associated with reappearance of proteinuria at 52 weeks while none of the patients in which suPAR went down after remission experienced a recurrence of proteinuria. When classifying patients based on suPAR levels after treatment, it was noted that patients in whom levels decreased to values <3000pg/ml, the reduction in urine protein excretion was significantly greater than that observed in patients who had suPAR levels greater than 3000pg/ml both in the short and long term (78 months). Taken together, these data suggest that although neither the baseline nor absolute changes in suPAR levels after treatment were associated with the response, relative changes and/or evidence of a reduction in suPAR levels to values lower than 3000pg/ml may be response predictors. In addition, persistence of high levels or the increase in levels following remission may be associated with the onset of recurrences. Baseline levels in patients from the European PodoNet cohort were significantly lower than those in the American cohort. Nevertheless, the cohorts are not comparable because, among other things, there were significant differences in age, ethnic distribution and, most importantly, very significant differences in the number of patients with FSGS with a genetic cause. In the European cohort, suPAR levels were significantly higher in patients with genetically-caused FSGS but so was proteinuria and serum creatinine. In addition, mean proteinuria and albumin levels in patients with non-genetically-caused FSGS indicates that the majority of cases did not have nephrotic syndrome at the time they were studied. This makes it difficult to associate suPAR levels with activity.
KEY CONCEPTS
1. Approximately 40% of patients with adult idiopathic FSGS are resistant to steroid and calcineurin inhibitor treatment, either initially or after one or several recurrences. Resistance to treatment is the most significant independent predictor of the development of progressive chronic kidney disease.
2. In patients with steroid and calcineurin inhibitor resistance, there is no treatment capable of modifying the clinical course of the disease in which the indication has been backed by appropriately designed clinical trials.
3. The indication for treatment with MMF, sirolimus, rituximab, apheresis or high-dose galactose is backed by very low levels of evidence and should be individualised following proper analysis of the risk associated with persistence of nephrotic syndrome activity.
4. In patients who develop steroid resistance following one or several recurrences after a good initial response, it would probably make sense to investigate pharmacodynamic causes for resistance associated with overexpression of glycoprotein P.
5. Although the expected prevalence is very low, in young adult patients with resistance to steroids and calcineurin inhibitors, identification of the association of the p.R229Q podocin variant and a pathogenic heterozygous mutation at NPHS2 may be useful for not prescribing immunosuppression, for advising patients who plan on having children and for selecting possible family donors in the case of kidney transplant.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Aetiological classification of focal segmental glomerulosclerosis
Table 2. Aetiological classification of focal segmental glomerulosclerosis
Table 3. Results of the main observational studies in which the efficacy of treatment with glucocorticoids in idiopathic focal segmental glomerulosclerosis is analysed
Table 4. Results of the main observational studies in which the efficacy of cyclosporine A in steroid-resistant idiopathic focal segmental glomerulosclerosis is analysed
Table 5. Results of the main studies in which the efficacy of mycophenolate in the treatment of steroid and calcineurin inhibitor-resistant focal segmental glomerulosclerosis is analysed.