En los últimos años se ha generado un debate sobre el rango de función renal normal y el ritmo de progresión de la enfermedad renal en el anciano. En esta revisión analizamos, basándonos en los resultados del estudio Ancianos con enfermedad renal crónica del Hospital General de Segovia, los factores de mal pronóstico asociados a esta enfermedad: proteinuria, episodios de fracaso renal agudo y de insuficiencia cardíaca, y el papel del ácido úrico. Los ancianos con enfermedad renal crónica que presenten estos factores de mal pronóstico serían los que se podrían beneficiar del seguimiento por Nefrología.
In the last few years a debate has emerged on the range of normal renal function and the rate at which renal disease progresses in the elderly. In this review we analysed, on the basis of the results of the study Ancianos con enfermedad renal crónica del Hospital General de Segovia (Elderly people with chronic kidney disease of the Hospital General de Segovia), the poor prognosis factors associated with this disease: proteinuria, episodes of acute renal failure and heart failure, and the role of uric acid. Elderly people with chronic kidney disease who present these poor prognosis factors may benefit from follow-up by Nephrology.
Content
It has been demonstrated in experiments on animals and clinical research in humans that the glomerular filtration rate (GFR) and the effective renal plasma flow experience a drop with age in the absence of renal disease.1-5 The Baltimore study showed a mean decrease in the GFR (estimated by creatinine clearance) of 0.75ml/min/year, while a third of the participants maintained stable creatinine clearance.6 Fliser et al. assessed the renal functional reserve by comparing 15 healthy, normotensive subjects, with a median age of 26 years (range 23 to 32) with 10 elderly subjects who had a median age of 70 years (range 61 to 82) and found that the GFR, estimated by inulin clearance (IC), was significantly lower in elderly subjects compared to the younger group (IC: 102 vs. 122ml/min), although the GFR estimated by IC in older people remained within the normal range.4 Later, another study that included 68 people also found that the GFR in elderly people was only slightly lower when compared to a control group of young people, and furthermore, that this decrease in the GFR was closely linked to the existence of associated conditions, such as high blood pressure (HBP) or heart failure (HF).5
The findings of this study demonstrate that healthy elderly people do not experience a serious deterioration in renal function4-6 and they call into question the strongly held belief that renal function decreases inexorably in elderly people.7
Moreover, the KDOQI Guidelines define/classify chronic kidney disease (CKD) by dividing it into five stages based on the GFR and/or presence of kidney damage (haematuria, proteinuria, abnormal imaging tests). The Guidelines also consider that the different stages of CKD are valid for people of all ages.8 There is, however, a lot of debate about what the range of normal renal function in elderly people is, and if a reduced GFR in elderly people is due to a physiological aging process or, on the contrary, it is due to intrinsic renal disease.
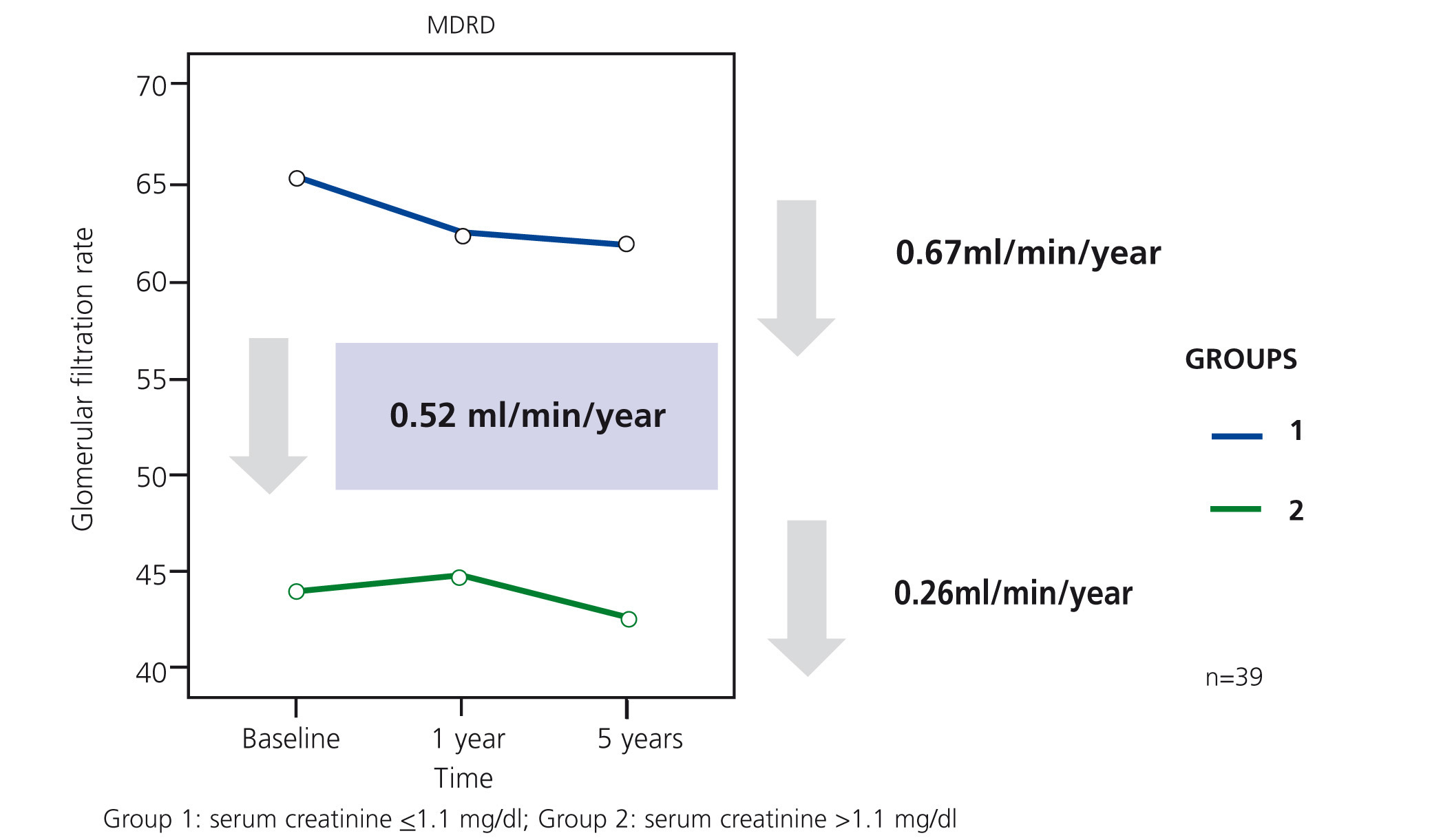
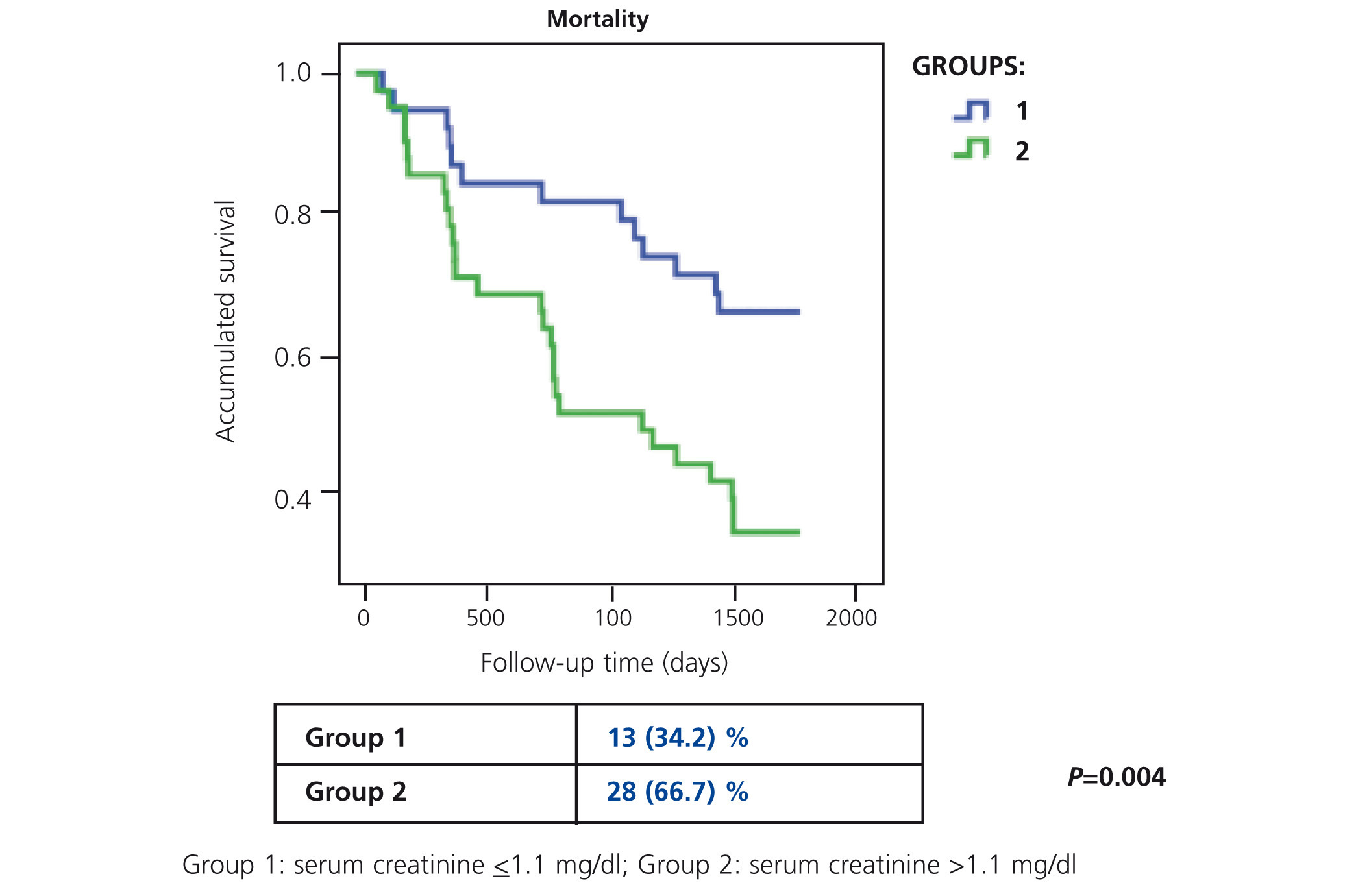
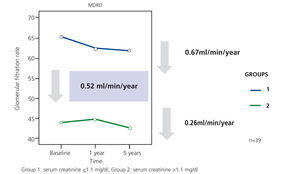
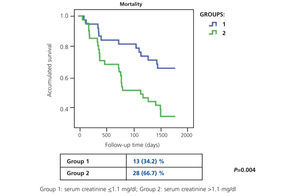
In our study, Ancianos con enfermedad renal crónica del Hospital General de Segovia, we describe the natural progression of renal function in two groups of patients followed up for a five year period:9 in this study, there was a slow mean decrease in renal function over time of 0.52ml/min/year (Figure 1). In the first group (patients with serum creatinine ≤1.1mg/dl without proteinuria), the mean decrease was 0.67ml/min; none of these patients progressed to end-stage renal disease (ESRD). In the second group of patients, with serum creatinine >1.1mg/dl, the mean decrease was 0.26ml/min/year; only two patients progressed to ESRD, although in no case was renal replacement therapy (RRT) performed. The lower decrease in the FG in subjects who had worse baseline renal function can perhaps be explained by the fact that most patients in the second group died before the kidney disease progressed to a terminal stage (Figure 2). The results of our study are in keeping with other previous studies in which it has been confirmed that the risk of these patients dying is greater than progression to TKD.10 In the study by Eriksen and Ingebretsen, 31% of patients died, compared with 2% who underwent RRT.11
With regard to the blood pressure control, it is known that a reduction in blood pressure is associated with a slowing of renal damage. In our work, most of the elderly people studied had blood pressure figures <140/90mmHg, leading us to speculate that this control may have had an influence on the renal function of these elderly subjects.9
In this review, we analysed the factors that could be involved in a worse CKD prognosis in elderly patients on the basis of the results obtained in our study: proteinuria, acute renal failure (ARF) episodes, episodes of HF and uric acid (UA).
PROTEINURIA: PROGNOSIS VALUE IN THE PROGRESSION OF RENAL FUNCTION AND IN MORTALITY IN THE ELDERLY
The presence of proteinuria is a fundamental sign of renal damage.12 Several previous studies have shown that proteinuria is a major risk factor for renal dysfunction and cardiovascular mortality.13,14 A recent meta-analysis that analysed nine cohorts of the general population and eight cohorts of high risk patients, which included more than one million people, found that decreased GFR coupled with the presence of albuminuria is associated with a high risk of TKD development, independently of other traditional cardiovascular disease risk factors.15
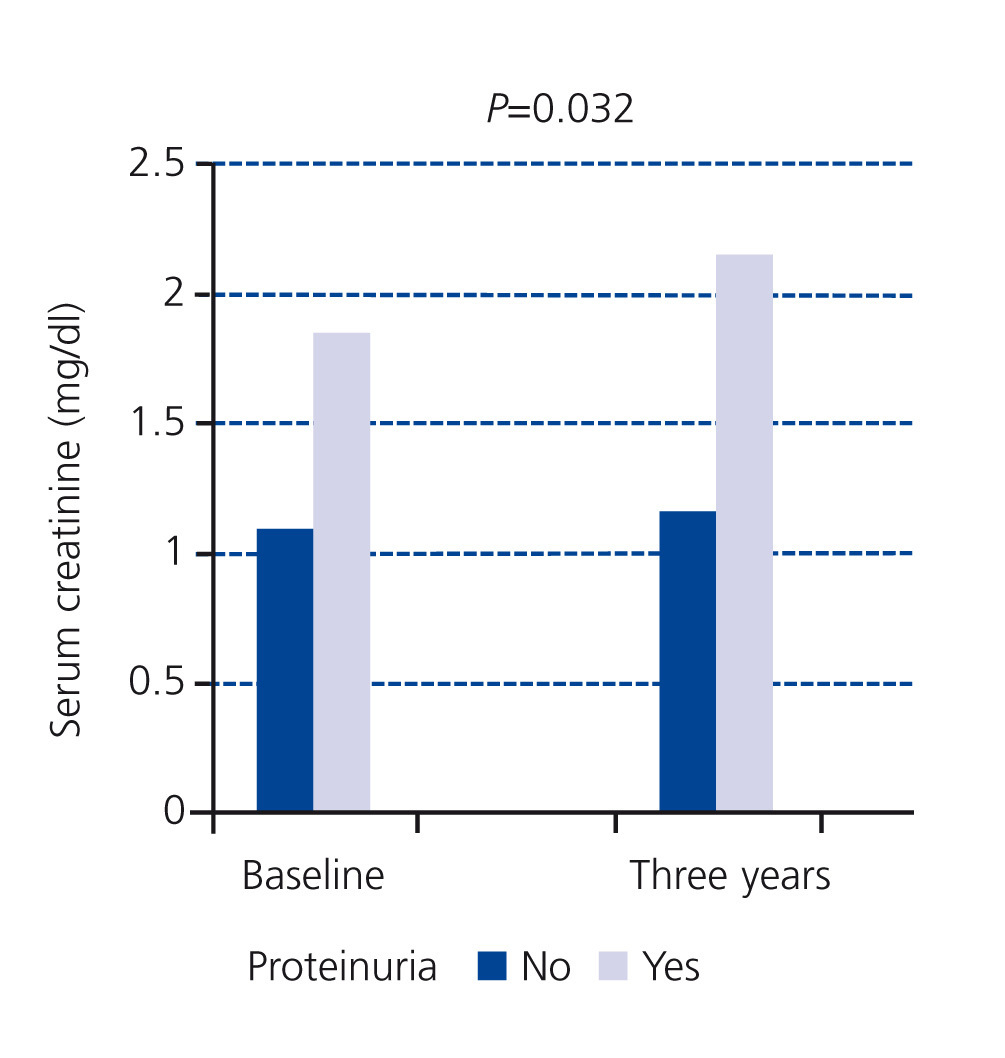
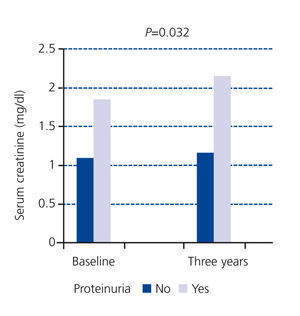
In our study, at the baseline cut-off, patients of the first group did not display proteinuria and only 13% of patients in the second group had proteinuria greater than 0.5g/day; we found that the serum creatinine figures of patients with proteinuria had significantly increased in three years (Figure 3) with respect to patients without proteinuria.16 As a practical result, this group of patients would benefit more from specific nephrological care.
One of the limitations of the KDOQI classification is precisely that proteinuria is taken into account for defining the least severe stages (stages 1 and 2) and, nevertheless, in the most advanced stages (3-5), it is sufficient to have an estimated GFR (eGFR) below 60ml/min to define them, independently of whether or not they present proteinuria. Therefore, it is possible that renal function of patients with stage 1 CKD and proteinuria progresses earlier to TKD requiring dialysis than that of other patients in stage 3 without proteinuria. In our study, we found patients with serum creatinine within a normal range without proteinuria with a decreased GFR, who could be classified as stage 3 CKD simply due to having a GFR below 60ml/min and, nevertheless, none of these patients progressed to TKD in the follow-up period.
In our study, the existence of proteinuria also proved to be an independent risk factor of mortality, as demonstrated by Cox’s analysis carried out in the follow-up at three years (relative risk [RR]: 2.12, P=.03).16
Our results are consistent with those reported in the study by Hemmelgarn et al., which analysed a cohort of 920,985 adults, with a mortality rate of 3% at a mean 35 month follow-up; the authors concluded that the risk of death, of suffering myocardial infarction or terminal kidney failure associated with a given level of eGFR increased independently in patients with higher levels of proteinuria.17
EPISODES OF ACUTE RENAL FAILURE
ARF is a sudden loss of kidney function that results in the accumulation of waste products (increased serum creatinine levels), and dysregulation of the water-electrolyte balance.18 In elderly people, the presence of certain comorbidities, polypharmacy, and the fact that certain invasive diagnostic and therapeutic procedures (medications, contrasts and surgery) are practiced more frequently in this population group, have contributed to the increase the incidence of ARF.19-21 In fact, it is suggested that the actual epidemic in nephrology is ARF instead of CKD.21,22
Those over 65 years of age are at risk for non-recovery of renal function after suffering an episode of ARF and even of progression to advanced CKD.21 A meta-analysis reveals that 31% of elderly people did not recover renal function after an episode of ARF, compared with 26% of younger patients.23
Various studies provide evidence that suffering ARF, the severity of the latter (requirement for dialysis) and its frequency (more than one episode) are associated with progression to chronic kidney disease.24-27 Other studies demonstrate that both the severity and the duration of ARF are factors related to mortality.28,29 In a specific study conducted in elderly people, Ishani et al. observed that patients with ARF episodes, particularly if they already had chronic renal damage, are more at risk of developing TKD than patients who have not suffered ARF episodes. The authors suggest that ARF may accelerate the progression of renal disease. This study also demonstrates an absolute mortality risk at two years of 29% in elderly subjects with ARF compared to those who did not suffer it.30
Another study has shown that even reversible ARF episodes with rapid recovery of renal function or patients with small increases of serum creatinine are associated with a higher risk of developing CKD (RR: 1.91) and mortality (RR: 1.50).31
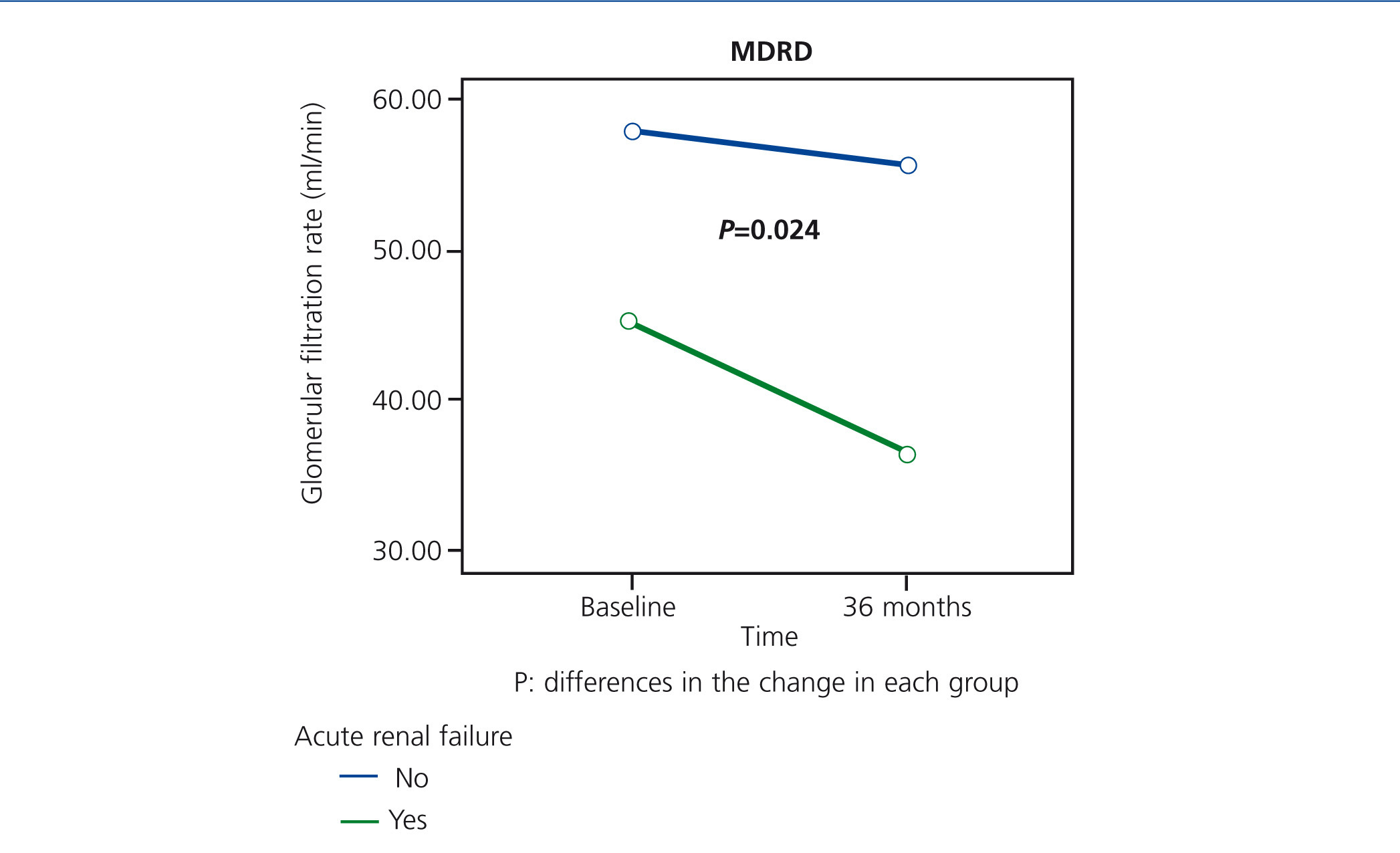
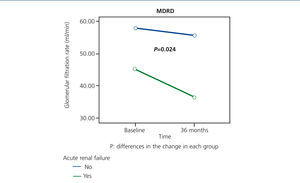
From the data of our study, approximately one quarter of patients who were still alive within 36 months of being recruited had an episode of ARF during a period in which they were hospitalised. Although their condition was reversible - functional aetiology and no requirement for dialysis treatment, patients with ARF had a greater decrease in renal function over time (Figure 4), and 25% of these patients died during the episode, confirming the high morbidity and mortality associated with this syndrome.32 According to these data, the elderly people that presented an episode of ARF would be a subgroup of patients that would benefit from follow-up in nephrology department.
EPISODES OF HEART FAILURE
Literature describes HF as the most common cause of hospitalisation in patients over 65 years of age.33 In Spain, the estimated prevalence of HF is around 7-8% in the general population aged 45 years or older (with a mean age of 64 years), and 1% of total hospitalisation rates correspond to decompensated HF episodes (with a higher average age).34 Among the aetiopathogenic mechanisms involved in the onset of HF in elderly patients, we can mention the changes associated with the aging process (progressive increase in left ventricular mass, loss of aortic elasticity, increase in the collagen content of a normal heart)35,36 and also, the association with diseases such as HBP, ischaemic heart disease (IHD) and heart valve disease.34
In our study, patients with a history of HF at baseline also had worse renal function.37 Furthermore, HF was the main cardiovascular event during follow-up, above IHD and stroke. 20% had an episode of HF which could be explained by the fact that it was a very aged population and also because HBP was a disease with high prevalence (over 80%). On analysing the presence of HF by groups, although the differences did not reach statistical significance (either due to the limited number of patients studied or the time period studied), patients in group 2 suffered more episodes of HF.9
URIC ACID
UA is a waste product of purine metabolism.38 Hyperuricaemia, defined by UA levels >6.5 or 7mg/dl in men and >6 mg/dl in women, is a finding that can be observed when renal function deteriorates.39,40 In observational studies, the UA level has been considered an independent risk factor for developing CKD,41,42 something that has also been demonstrated recently for ARF.43,44 In a specific study on the elderly Chinese population, which included 1182 people ≥65 years of age, it was found that a high percentage had hyperuricaemia; an association was also found between hyperuricaemia and other cardiovascular risk factors.45
There is much debate regarding the role of UA both in the onset and in the progression of kidney disease. A cross-sectional study was unable to determine which event occurs first, the increase in UA or kidney disease.40 However, some longitudinal follow-up studies show that UA is a risk factor for developing CKD over time.46,47 In the same vein, other studies also show that the treatment of hyperuricaemia with allopurinol reduces the risk of kidney disease progression48 or improves endothelial dysfunction.49
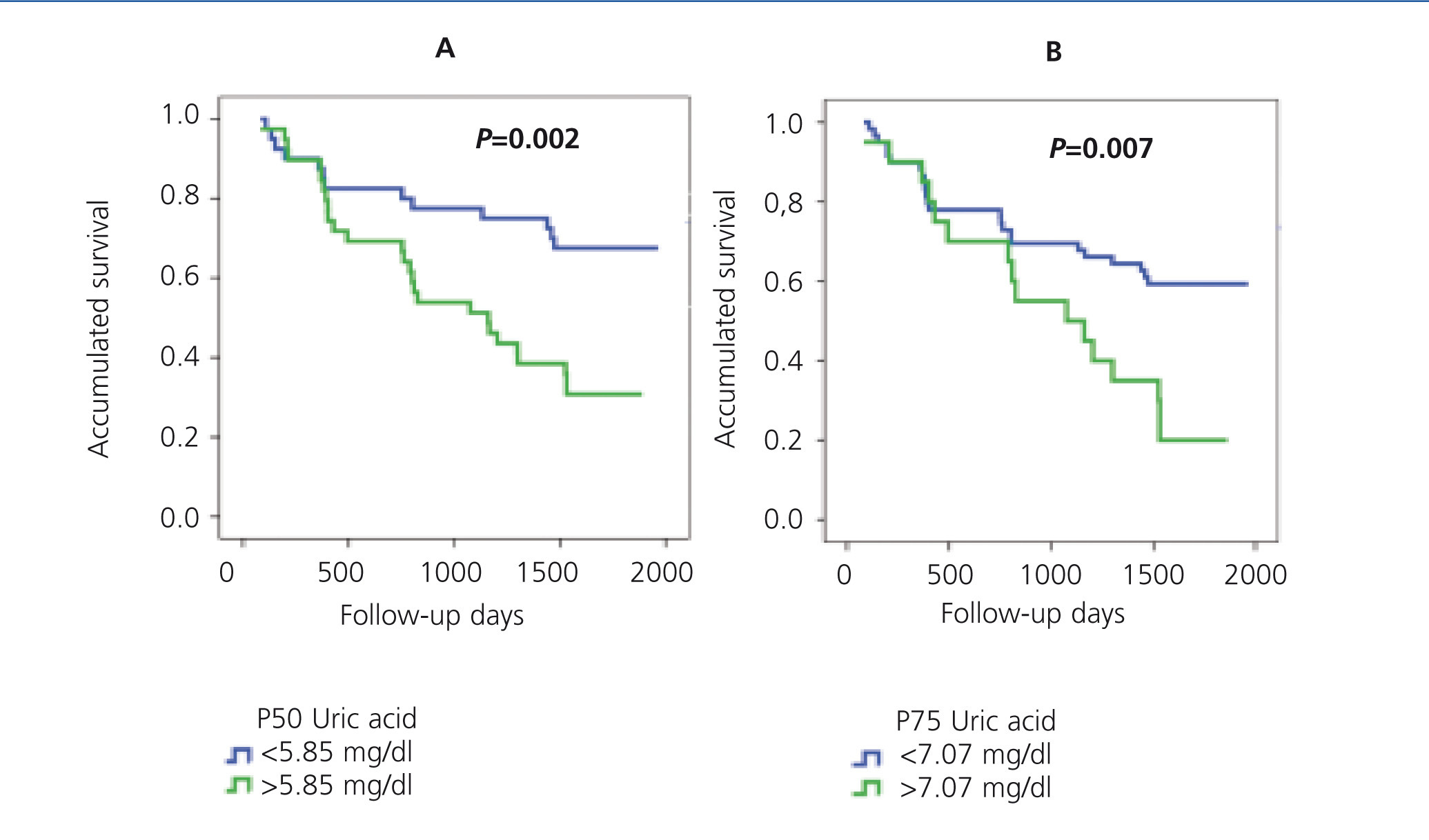
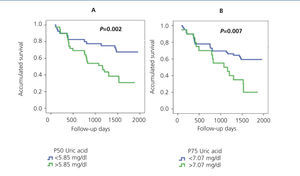
It has also been demonstrated that UA levels act as a risk factor for mortality, both cardiovascular and general.50,51 In our study Ancianos con enfermedad renal crónica del Hospital General de Segovia, UA was observed as an independent risk factor both for the development of ARF (RR: 2.65, P=.027)32 and for general mortality (RR: 1.35, P=.000).52 Figure 5 shows the Kaplan-Meier mortality curves according to the UA percentiles: patients with higher UA levels also had a worse degree of baseline renal function.52
In conclusion, elderly people with CKD who present poor prognosis factors, such as proteinuria, a previous history of acute renal failure or HF and hyperuricaemia, would be a subgroup of patients who would benefit from follow-up in Nephrology clinics.
KEY CONCEPTS
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Figure 1. Decrease in the glomerular filtration rate in survivors of the study on Elderly patients with chronic kidney disease at 5 years
Figure 2. Progression of mortality at 5 years of the study on Elderly patients with chronic kidney disease for groups of different levels of baseline renal function
Figure 3. Impact of proteinuria on serum creatinine progression at three years
Figure 4. Impact of acute renal failure on the progression of renal function at 36 months
Figure 5. Kaplan-Meier mortality curve according to P50 (A) or P75 (B) of uric acid