El acceso vascular funcional es un requisito previo para el tratamiento renal sustitutivo en pacientes con enfermedad renal crónica. Las fístulas autólogas se consideran superiores a las prótesis vasculares y los catéteres venosos centrales; sin embargo, las fístulas no están exentas de problemas. Las trombosis de la fístula autóloga se han convertido en un reto en la práctica clínica de nefrología, con importantes implicaciones clínicas para pacientes en diálisis. Varios estudios han informado sobre la viabilidad y la tasa relativamente alta del éxito clínico del abordaje endovascular de fístulas trombosadas en los últimos años. Sin embargo, como las repetidas intervenciones suelen ser necesarias para lograr la supervivencia a largo plazo del acceso, el mantenimiento de una fístula anteriormente trombosada podría ser una política muy cara. Los objetivos de este artículo son proporcionar al lector una idea de los múltiples enfoques endovasculares para fístulas autólogas trombosadas, teniendo en cuenta su eficacia clínica y las implicaciones financieras.
Functional vascular access is a prerequisite for adequate haemodialysis treatment in patients with end-stage renal disease. Autogenous arteriovenous fistulae are considered superior to synthetic grafts and central venous catheters; however, fistulae are not without problems. Fistulae thrombosis has become a clinical challenge in nephrology practice, with relevant clinical implications for dialysis patients. Several studies have reported on the feasibility and relatively high-clinical success rate of the endovascular approach to thrombosed fistulae in recent years. However, as repeated interventions are usually required to achieve long-term access survival, maintenance of a previously thrombosed fistulae could be a highly expensive policy. The goals of this article are to provide the reader an insight into the multiple endovascular approaches for thrombosed arteriovenous fistulae, bearing in mind its clinical effectiveness and financial implications.
INTRODUCTION
The number of patients undergoing dialysis continues to increase as technology and patient survival improve in this patient population. Portugal has a higher incidence and prevalence of end stage-renal disease (ESRD) compared to most of other European countries. In 2011, a prevalence rate of 1662 patients per million of the population was registered by the Portuguese Society of Nephrology (10,409 patients underwent haemodialysis and 704 patients peritoneal dialysis in 2011). We know that vascular accesses for haemodialysis are plagued with multiple problems, the most common being infection and dysfunction. Although thrombosis of the vascular access is a relatively infrequent complication of autogenous arteriovenous fistulae (AVF), as current clinical practice guidelines recommend that at least 65% of ESRD population should have a functional AVF as a permanent dialysis access,1,2 AVF thrombosis has become a clinical challenge in our nephrology practice, with relevant clinical implications for dialysis patients. The challenge of determining the most effective treatment for thrombosed AVFs is paramount in the minds of the nephrologists. The goal of this article is to provide the reader an insight into the endovascular approaches of thrombosed AVFs, bearing in mind its clinical effectiveness and financial implications.
PRE-PROCEDURAL PATIENT ASSESSMENT
Before the thrombectomy procedure it is important to determine whether the patient has a history of significant cardiac or pulmonary disease. Patients who have a history of right-sided heart failure or pulmonary hypertension are not good candidates for an endovascular thrombectomy procedure since fragments of thrombus can escape from the AVF and travel to the lungs as pulmonary emboli during the procedure.3,4 In these particular clinical scenarios, therapy should be individualized, taking into account the risk-benefit of endovascular thrombectomy.
The patient’s vascular access should be examined before draping the extremity. Fistula thrombosis is a clinical diagnosis characterized by the absence of flow in the AVF. Physical examination provides additional information that is of the utmost importance for the interventionalist since different endovascular approaches are used for AVFs with inflow, outflow, co-existing inflow–outflow problems or AVF thrombosis. Although clinical signs of infection should always be inspected, local inflammation and pain immediately upstream from the stenosis occur frequently in recently thrombosed native fistulae (phlebitis), and the diagnosis of infection is not easy to make.
There are few contra-indications to percutanous declotting. Local infection is the main clinical contraindication. Huge clot burden (>100cc) and large aneurysms with old wall-adherent thrombi are both clinical and technical contraindications because safe removal of thrombi in such conditions is extremely difficult and hazardous to the patient. Immature AVF never previously used for haemodialysis, once considered as a technical contraindication, has recently been revisited by Miller et al.,5 reporting a highly success rate of endovascular salvage of immature clotted AVFs.
PERCUTANOUS THROMBECTOMY PROCEDURE
Throughout the past two decades, there has been a plethora of published reports describing numerous percutaneous techniques for the treatment of thrombosed haemodiaysis grafts and AVFs.6-19 These techniques can be divided into two broad categories; one group uses thrombolytic agents, and the other uses mechanical thrombectomy technique. The category of mechanical thrombectomy techniques includes balloon thrombectomy, mechanical thrombectomy devices and thromboaspiration.
Basic Technique
Percutaneous declotting of a dialysis AVFs is an outpatient procedure. Patients are monitored by a nurse with pulse oximetry, blood pressure measurement, and electrocardiography. Fentanyl and/or midazolam can be administered intravenously for conscious sedation. Puncture sites are anesthetized with lidocaine. Systemic heparinization with 5000UI of heparin is initiated prior to the procedure. Prophylactic antibiotics are recommended by some authors.13
Although the technique for declotting a polytetrafluoroethylene graft can be standardized easily, clotted AVFs result in a wide range of difficulties: (a) the thin venous wall is more difficult to cannulate; (b) the anatomy is irregular, with the presence of collaterals veins; (c) the locations of the stenosis can occur anywhere, and are difficult to traverse; (d) a large volume clots can be encountered; and (e) aneurysms are more frequent than in polytetrafluoroethylene grafts.
The technique for declotting a thrombosed AVFs has been well described by Turmel-Rodrigues et al.13 There are four basic steps to perform during a percutaneous thrombectomy procedure: (a) physical examination is essential to choose the best site for initial catheterization; (b) an initial venogram to evaluate the central and peripheral veins; (c) removal of the thrombus from the vascular access; and (d) treatment of all significant stenoses. An underlying stenosis is unmasked in the great majority of cases. In typical cases, an initial introducer sheath is placed a few centimeters from the anastomosis using an antegrade approach to treat the venous outfow. A catheter is pushed over a wire up to the superior vena cava and then slowly pulled back while contrast medium is injected under fluoroscopy to localize the downstream extension of the thrombosis. The fistula is abandoned at this stage if the venous outflow cannot be traversed or recanalized. A second introducer is placed with a retrograde approach in the direction of the arterial inflow. The fistula is abandoned if it is impossible to traverse the arteriovenous anastomosis with the guidewire. Occasionally, when the stenosis is clinically located a few centimeters from the wrist anastomosis, with no evidence of concomitant outflow stenosis, a single retrograde approach from the vein at the elbow is sufficient to treat the whole fistula. Once access to both the arterial inflow and venous outflow is guaranteed with a guidewire, thombi on the venous side are removed first, before the thrombi on the arterial side. Conventional, high-pressure or cutting balloon angioplasty should be undertaken to remove causative stenotic disease. On completion, both physical examination and fistulogram are performed to visualize the flow from arteriovenous anastomosis to the superior vena cava. Vascular sheaths are removed and haemostasis is achieved by manual compression or using a purse-string suture.20
Occasionally, an AVF might clot with minimal or no thrombus. At other times, there is moderate-to-severe thrombus burden that accompanies AVF clotting. While percutaneous balloon angioplasty to correct the underlying stenosis might be all that is needed to declot a fistula with no thrombus, endovascular thromboaspiration is required to successfully declot a fistula with moderate thrombus and surgical referral is advisable in the presence of excessive thrombus burden.
Thrombolysis
The introduction of mechanical thrombectomy devices has reduced the popularity of thrombolytic therapy. Urokinase, streptokinase, and tissue plasminogen activator (t-PA) have all been used for infusion thrombolysis.6,21,22 To achieve optimum outcome, anterograde vascular access is obtained as close to the arteriovenous anastomosis as possible. Thrombolytic therapy is then administered via a multiple side hole catheter along the length of the fistula for between 3-24 hours, at doses depending on institutional protocol. To improve outcome, adaptations to the technique have been published: pulse spray thrombolysis, in which highly concentrated fibrinolytic therapy is injected as a high-pressure spray directly into thrombus for 15-20 min, thereby reducing procedural time.6 Due to modest success rates, thrombolysis is more frequently used in combination with mechanical thrombectomy to maximize clot clearance and reduce procedural times.
Mechanical thrombectomy
Several methods of mechanical clot dissolution have been published. Balloon thrombectomy was the first published “purely mechanical” percutaneous technique used for declotting thtombosed polytetrafluoroethylene grafts.23 This approach was accomplished using a variety of devices to macerate, dislodge or sweep thrombus from the occluded graft into the central venous circulation. This controversial technique was a milestone in the history of haemodialysis access declotting. From this experience gained in grafts, some teams successfully used this technique of deliberated pulmonary embolizations of clots when the volume of thrombus in the AVF was presumed to be equivalent to the encountered in grafts.22 Trerotola and colleagues stopped using this technique soon after, when they experiences a casualty.8 Other casualties have since been reported in the literature.3
A wide range of mechanical thrombectomy devices have been used in the treatment of failed AVFs. The Arrow-Trerotola PTD (Arrow International, Reading, PA, USA) is a rotating nitinol basket available in 5 and 7 F configurations. A handheld battery powered motor results in a basket rotation speed of approximately 3,000rpm, macerating thrombus into particles of 1 to 3mm diameter.24,25 Thrombus fragments are then aspirated manually using the introducer sheath side port. Simple rotation of a 5 F mini-pigtail catheter (Cook Europe, Bjaeverskov, Denmark) can be used to remove thrombus from occluded AVFs.26 The Oasis recirculation catheter (Boston Scientific/Medi-Tech, Natick, MA) is available in 6 and 8 F systems and uses a standard angiographic pump injector.27 The Hydrolyser (Cordis, Miami, FL, USA) is a dual lumen 6 or 7F catheter with a distal side hole and rounded tip and requires the use of a conventional contrast injector to administer saline retrogradely.9,28-30 The AngioJet catheter (Possis Medical, Minneapolis, MN, USA) is available in 4 to 6F systems uses a specialized pump drive system that creates high pressures.14,31 The Amplatz thrombectomy device (ATD; Microvena, White Bear Lake, MN, USA) consists of a sharp blade that is rotated at 150,000rpm by a compressed gas driven turbine, within a protective metal capsule. Thrombus is macerated by the rotating blade and dispersed into the bloodstream as microscopic particles.15 Manual catheter-directed thromboaspiration is a popular technique in France and Spain that uses a straight 7 to 9F end-hole catheter (Guider; Boston Scientific, Natick, MA, USA; or Vista Bright Tip; Cordis, Miami, FL, USA) to remove thrombus by manual suction.13,32
Success
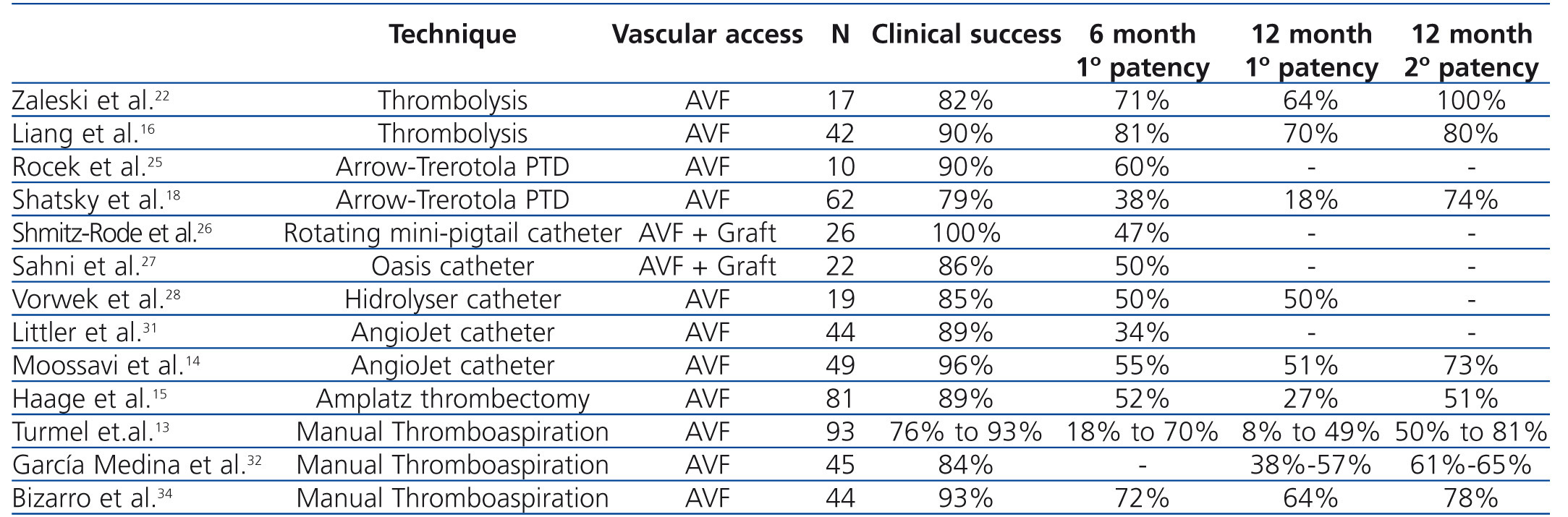
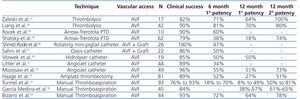
No single percutaneous thrombectomy technique has been proven to be more efficacious than other methods (Table 1). Limited data exist regarding outcome of declotting procedures in AVFs using thrombolysis alone. Zaleski et al.22 treated thrombosed AVFs with urokinase thrombolysis and balloon angioplasty and reported a procedural success rate of 82%. Primary patency rate of 64% was achieved at 12 months. Rocek et al.25 reported a 90% clinical success rate in 10 patients treated using the Arrow-Trerotola PTD. The 6-month primary patency rate was 60%. Shmitz-Rode et al.26 reported 100% clinical success rate in 15 AVFs and 11 grafts using the “rotating mini-pigtail catheter”, with a primary patency rate of 47% at 6 months. Sahni et al.27 treated 23 thrombosed accesses (5 AVFs and 17 grafts) using the Oasis catheter with a success rate of 86% and a primary patency rate of 50% at 6 months. Vorwek et al.28 reported a clinical success rate of 85% in 19 clotted AVFs and a primary patency rate of 50% at 12 months using the Hydrolyser catheter. Littler et al.31 published the outcomes of AngioJet thrombectomy in 44 occluded AVFs, with a technical success of 89% and a primary patency rate of 34% at 6 months. Similar results were confirmed by Moossavi et al.14. Recently, Yang et al.33 reported a higher success rate using the Arrow-Trerotola PTD, compared to the AngioJet catheter. However, comparable patency rates were obtained at one year of follow-up. A single study examining the clinical outcome of the ATD in occluded AVFs demonstrated a success rate of 89% and a primary patency of 27% at 12 months.15 Turmel-Rodrigues et al.13 reported the results of manual catheter-directed thromboaspiration technique, with a success rate of 93% for forearm and 76% for upper arm AVFs and a primary patency rate of 49% and 9% in the forearm and upper arm at 12 months, respectively. Similar results were reported by García Medina et al.32 and Bizarro et al.34.
Intervention studies on thrombosed autogenous fistulae have predominantly appeared after the year 2000. A few have compared endovascular with surgical repair, but none was randomized. The results were comparable with the outcome of endovascular treatment in terms of primary success rate (90% versus 89%), but 1-year primary (74% versus 40%) and secondary patency rates (87% versus 72%) were higher.35 However, most studies on surgical thrombectomy of AVFs concerned forearm accesses with the creation of a new, more proximally located arteriovenous anastomosis.35-37
Complications
The most frequent procedure-related complication associated with angioplasty of the dialysis vascular access is some type of venous rupture. This complication has been reported to represent 70–75% of all complications.38 Local complications, such as secondary bleeding and pseudoaneurism from the introducer sheath and vessel injury/disruption do occur in daily clinical practice,39 but probably are underreported in the literature.
There are few significant procedure-related complications. The risk of pulmonary embolism is theoretically greater with autogenous AVFs than with polytetrafluoroethylene grafts.40, 41 However, clinically silent pulmonary embolism probably occurs in many patients treated by these methods.40 Arterial embolization can result from clot fragmentation at the arterial anastomosis by catheter or guidewire manipulation, vigorous injection of contrast material, or balloon angioplasty of residual thrombus. However, the reported frequency of arterial embolization has been low (0-7%).42 Major hemorrhagic complications requiring additional treatment are reported in 1-7% of cases in these series.42 Some experts advocate the use of prophylactic antibiotics due to the risk of infection.13
COSTS
Probably, no topic has been so underreported in the nephrology literature as the analysis of costs of endovascular procedures for haemodialysis AVF failure. Vascular access costs may account for approximately 10% of the total cost of health care of haemodialysis patient population, with patients dialyzed with a catheter incurring the highest costs.43,44 It is well known for interventionalists that mechanical thrombectomy devices are expensive (e.g. Arrow-Trerotola PTD, $600; Hydrolyzer, $600; Oasis, $600). However, they are not the ones responsible for the high expenditure of endovascular procedures. The amount of resources required for endovascular interventions vary among vascular access centers with different endovascular salvage procedures (Table 2). Therefore, cost analysis must take into account all the devices employed during the procedure (p.e. guidewires, balloon angioplasty, stents) the pharmacy (e.g. antibiotics, heparin, thrombolytics) and radiology costs (e.g. angiography suite, contrast media), professional fees and additional overhead expenses.
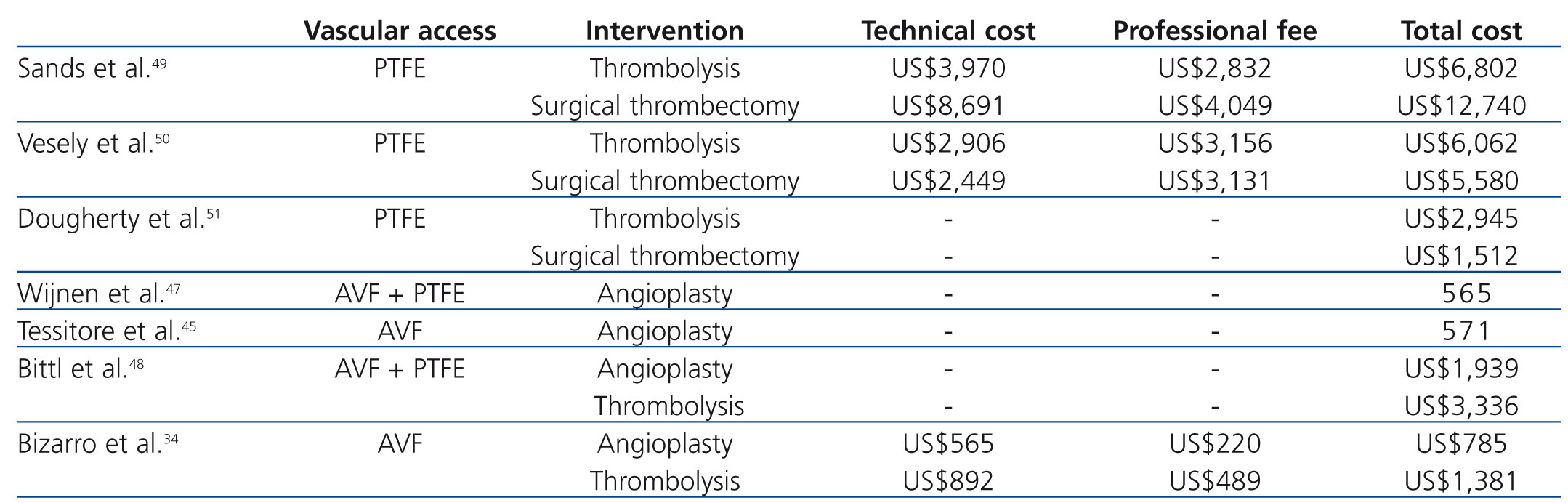
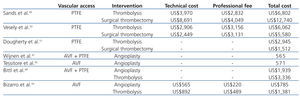
Published findings regarding the economic value of vascular access surveillance revealed that adding access blood flow surveillance to clinical monitoring of grafts and AVFs may reduce thrombosis rates and costs.45-47 Bittl et al.48 recently reported an economic analysis of angiography and pre-emptive angioplasty to prevent haemodialysis access thrombosis. They observed that pre-emptive angiographic management of malfunctioning nonthrombosed haemodialysis accesses may represent a less efficient use of healthcare resources than increasing the number of patients with AVFs. To our knowledge, cost-effectiveness analyses of endovascular interventions for thrombosed haemodialysis accesses have been performed mainly in patients with clotted prosthetic grafts.49-51 Sands et al.49 performed a retrospective analysis comparing the clinical success and the costs of pharmacomechanical thrombolysis of occluded grafts with surgical thrombectomy. Hospitalization was required for 85% of the cases of surgical thrombectomy and in 17% of cases of pharmacomechanical thrombolysis. Hospital charges and physician costs were obtained for each procedure. Clinical success was similar between lysis and surgical procedures. Hospital, physician and total charges were significantly lower in the lysis group than in the surgical control (US$6,802 versus US$12,740). Vesely et al.50 prospectively randomized 20 patients with clotted grafts either to pulse-spray thrombolysis plus angioplasty, or surgical thrombectomy. Thrombolysis and surgical thrombectomy were performed as outpatient procedures in almost of the cases. The technical costs, professional fees, and all other associated costs were obtained. The authors concluded that endovascular thrombolysis and surgical thrombectomy were comparable in cost (US$6,062 versus US$5,580), and the technical success and patency rates were also similar. Dougherty et al.51 performed a similar study among 80 patients with clotted grafts. The mean cost of treatment (including room and supply costs but not professional fees) was significantly higher for the endovascular group than for the surgical group (US$2945 versus US$1512). In addition, high rate of technical failure necessitating surgery was observed in the endovascular group. To our knowledge, only recently the costs of percutaneous thrombectomy of clotted AVF were reported in the literature.34,48 Bizarro et al.34 reported a cost analysis of the use of manual catheter-directed thromboaspiration for the treatment of thrombosed AVFs. A comprehensive measurement of total vascular access care-related costs was obtained. The authors reported that the cost of maintenance of thrombosed AVFs by endovascular means was high. The mean total expense of the percutaneous thrombectomy procedure was US$1,381 and for percutaneous transluminal angioplasty was US$785. At one year of follow-up, the mean cumulative cost of vascular access care was US$2,504 per patient-year at risk. The mean cost was greatest for patients with brachiocephalic AVFs (US$3,578) than for patients with forearm AVFs (US$1,604). In comparison, in the Bittl et al. study48 procedure costs were approximately two times higher (angioplasty, US$1,939; percutaneous thrombectomy, US$3,361). Possible explanations for the differences observed in these two studies are: (a) in the first study,34 the investigators used manual catheter-directed thromboaspiration technique whereas Bittl et al.48 used the AngioJet catheter for thrombosed AVFs; (b) stents were not used in the first study,34 whereas Bittl et al.48 placed stents for several indications and; (c) physician billing differ among countries.52
Percutaneous thrombectomy is an effective policy to treat thrombosed failed AVFs. Yes, but an expensive one. Do we, nephrologists, have other cost-effective approaches to deal with failed AVF? Does surgical thrombectomy may overcome the high financial burden of percutaneous thrombectomy, with similar success rates? Does the abandon of failed AVFs toward the creation of a new permanent accesss still an option? To our knowledge, clear-cut data has not been published answering these questions. Recenlty, Miller et al.5 evaluated the efficacy, functionality, and cost associated with the use of percutaneous techniques for the salvage of thrombosed immature fistulae. All fistulae had thrombosed following access creation and had never been used for haemodialysis. The authors concluded that endovascular techniques yield significant cost savings over access abandonment. Coentrão et al.53 compared two distinct policies for the maintenance of vascular access in prevalent haemodiaysis patients with thrombosed AVFs: percutaneous thrombectomy versus central venous catheterization to bridge the interval until a new AVF is suitable for cannulation. The authors observed that AVF salvage by endovascular therapy led to a near two-fold reduction in access-related expenses; the added costs associated with the procedure itself was completely offset by the saving associated with lower surgical visits, access dysfunction, and hospitalizations.
Taking into consideration the published high success rates and the costs of percutaneous thrombectomy of occluded autogenous fistulae, nephrologists, Medical Societies and National Health Services face a new era of vascular access care. If the final decision is to treat failed AVFs, intensive efforts should be undertaken to universalize these interventions.
Key concept
Several studies have reported on the feasibility and relatively high-clinical success rate of the endovascular approach to thrombosed autogenous arteriovenous fistulae in recent years. However, maintenance of a previously thrombosed arteriovenous fistulae require repeated interventions and thus could be a highly expensive policy. Further prospective cost-effectiveness analyses comparing different thrombectomy procedures (endovascular versus surgery) and distinct approaches for the maintenance of functional autogenous fistulae (pre-emptive angioplasty versus percutaneous thrombectomy) need to be carried out.
Conflict of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Results of dialysis autogenous arteriovenous fistula (AVF) declotting procedures
Table 2. Costs of endovascular procedures for dysfunctional and thrombosed haemodialysis autogenous arteriovenous fistula (AVF) and polytetrafluoroethylene graft (PTFE)