Antecedentes: Los pacientes con nefropatía endémica de los Balcanes (NEB) en hemodiálisis necesitan una dosis más elevada de eritropoyetina recombinante humana que los pacientes con otras enfermedades renales para poder mantener el nivel de hemoglobina deseado. Objetivos: Comparar la farmacocinética de la eritropoyetina beta administrada subcutáneamente a pacientes en hemodiálisis con NEB u otras enfermedades renales. Métodos: A 10 pacientes hemodializados con NEB y 14 pacientes hemodializados con otras enfermedades renales se les administró subcutáneamente eritropoyetina (EPO) recombinante humana (75 U/kg). El nivel de EPO en plasma previo a la dosis se sustrajo de todos los niveles que se obtuvieron tras administrarla. Los parámetros farmacocinéticos relevantes se calcularon tras el análisis farmacocinético no compartimental con ayuda de la aplicación Kinetica (Thermo Scientific, versión 5.0). Resultados: Aunque la concentración basal de EPO en el plasma era similar en pacientes con NEB (20,1 ± 10,3 U/l) y en pacientes con otras enfermedades renales (15,1 ± 8,1 U/l; p = 0,1964), se apreciaron diferencias significativas entre los grupos con respecto a la constante de eliminación (0,016 ± 0,006 vs. 0,026 ± 0,011 h-1; p = 0,020) y a la semivida de eliminación (50,24 ± 19,12 vs. 33,79 ± 18,91 h, p = 0,048). Estas diferencias resultaron igualmente significativas tras ajustar los valores en función de las características de los pacientes (edad, sexo, duración de la hemodiálisis, ferritina, hormona paratiroidea y uso de inhibidores de la enzima de conversión de la angiotensina). No obstante, no se observaron diferencias significativas entre los grupos en los siguientes parámetros ni antes ni después de efectuar los ajustes pertinentes: concentración máxima de EPO, tiempo en alcanzar la concentración máxima de EPO, área bajo la curva extrapolada al infinito a partir de la administración de la dosis, aclaramiento y tiempo medio de permanencia de la EPO. Conclusión: El análisis farmacocinético de la eritropoyetina beta reveló que la semivida de eliminación era significativamente más larga en los pacientes con NEB, hallazgo que debe ser confirmado en un estudio bien controlado con una muestra más grande.
Background: Balkan endemic nephropathy (BEN) hemodialysis patients require a higher dose of recombinant human erythropoietin for maintaining target hemoglobin level than patients with other kidney diseases. Objectives: Comparison of the pharmacokinetics of beta-erythropoietin given subcutaneously to hemodialysis patients with BEN or other kidney diseases (non-BEN). Methods: Recombinant human erythropoietin (75U/kg) was administered subcutaneously to 10 BEN and 14 non-BEN hemodialysis patients. The predose plasma level of erythropoietin (Epo) was subtracted from all postdose levels. The relevant pharmacokinetic parameters were calculated after noncompartmental pharmacokinetic analysis using Kinetica software (Thermo Scientific, ver.5.0). Results: Although basal plasma Epo concentration was similar in BEN (20.1±10.3U/L) and non-BEN (15.1±8.1U/L; p=.1964) patients, there were significant differences between the groups for elimination rate constant (0.016±0.006 vs 0.026±0.011 hr-1; p=.020) and elimination half-life (50.24±19.12 vs 33.79±18.91 hr, p=.048). These differences remained significant after adjustment for patient characteristics (age, sex, hemodialysis duration, ferritin, PTH and ACEI use). No significant differences between groups were found in maximal Epo concentration, time to maximum Epo concentration, area under the curve from time of dosing extrapolated to infinity, clearance, mean residence time of Epo between groups both before and after adjustment. Conclusion: Pharmacokinetic analysis of beta-erythropoietin detected a significantly longer elimination half-life in BEN than in non BEN patients. This finding needs to be confirmed in a well-controlled study with a larger sample size.
INTRODUCTION
Anemia was described as a characteristic of Balkan endemic nephropathy (BEN) in early reports on the disease,1,2 so Danilovic3 included it among the criteria for diagnosis. However, several authors reported no significant differences between anemia in BEN and other kidney diseases.4-6 Thus, although anemia in BEN patients deteriorates with progressive renal failure similarly as in patients with other kidney diseases, it was more severe in BEN patients on hemodialysis than in those with other kidney diseases.6,7
We recently showed that BEN hemodialysis patients required a higher dose of recombinant human erythropoietin for maintaining the target hemoglobin level than patients with other kidney diseases.8 Several factors may contribute to the weaker hematopoietic response to erythropoietin-stimulating agents (ESA). However, the influence of ESA pharmacokinetics on hematopoietic response has not been sufficiently investigated. Pharmacokinetics studies were mostly devoted to the pharmacokinetics of diverse ESA administered by different routes,9-13 as well as to the pharmacokinetics of ESA in various groups of chronic kidney disease patients and healthy persons.14-16
The aim of this study was to compare the pharmacokinetics of beta-erythropoietin (beta-Epo) given subcutaneously to BEN patients and patients with other kidney diseases (non-BEN) and to evaluate the factors influencing beta-Epo kinetics.
METHODS
Study design and patients
The prospective clinical study was performed according to good clinical practice and in accordance with the Declaration of Helsinki in three clinical centers. The Ethics Committee of the Clinical Center of Serbia evaluated and approved the study and the participants gave informed consent.
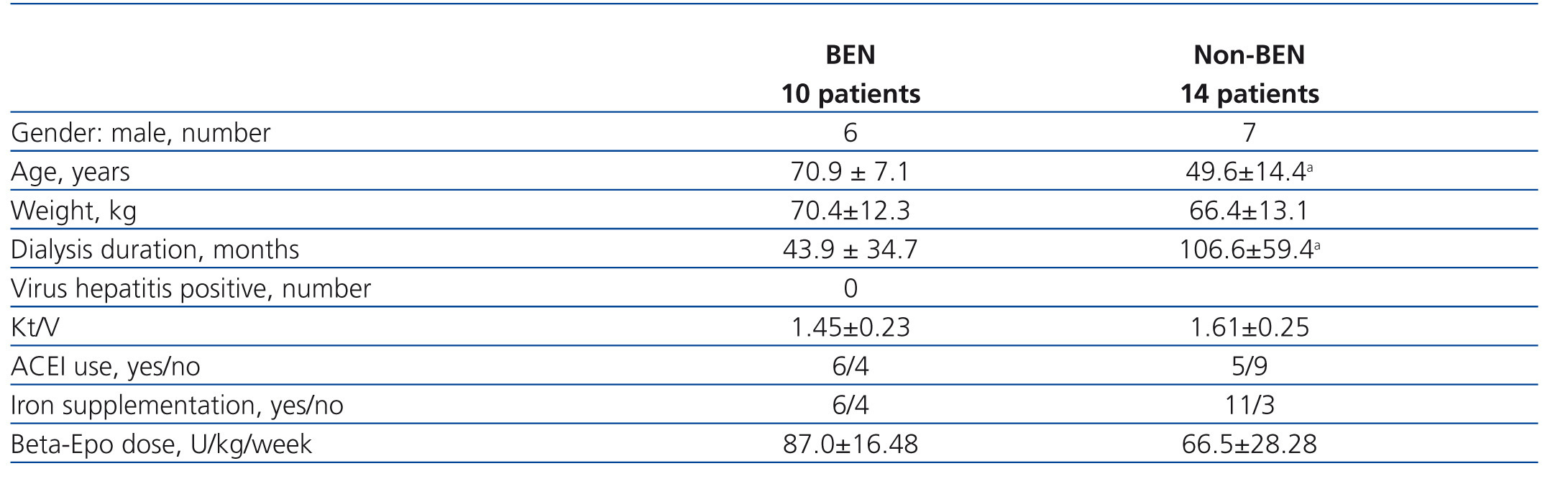
A total of 24 subjects was selected from the population of 256 hemodialysis patients according to the following inclusion criteria: stable individuals on regular hemodialysis, treated with beta-Epo for at least 1 year. Exclusion criteria were: treatment with ESA other than beta-Epo; anemia from causes other than chronic kidney disease - blood loss, malignancy, acute infection, hemolysis, major surgery; liver disease (twice or half normal values for transaminases); blood transfusion within one month before enrolment; severe psychiatric dysfunction or other limitations preventing the patient to give consent. Among the remaining 96 patients (40 BEN and 56 others) who met inclusion criteria, 10 with BEN and 14 with other kidney diseases were selected using systematic sampling i.e every 10th patient from each group was assigned for study. The group with other kidney diseases consisted of 8 patients with glomerulonephritis, 3 with hypertensive nephropathy, 2 with reflux nephropathy and 1 with polycystic kidney disease.
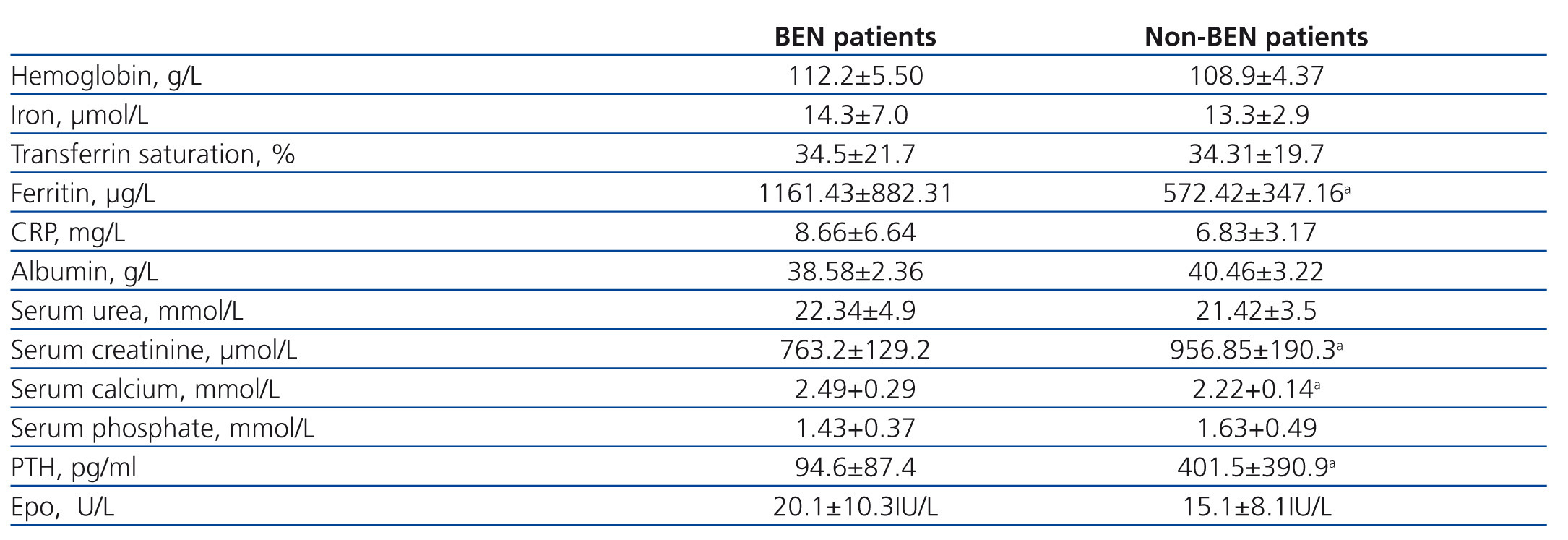
All patients were treated thrice weekly for 4-5 h by standard bicarbonate hemodialysis using polysulphone membrane dialyzers. Data on patients’ weight, blood pressure, use of angiotensin converter enzyme inhibitors (ACEI) and angiotensin II receptor antagonists (ARA II), hemoglobin, rHuEpo dose and iron treatment were obtained from medical records. Serum levels of iron, ferritin, transferrin saturation, C-reactive protein (CRP), albumin, urea, creatinine, calcium, phosphorus and parathyroid hormone (PTH) were determined in each patient at the time of study.
Beta-erythropoietin administration and blood sampling
All patients had been treated with beta-Epo (Epoetin-beta, Recormon®, Roche) for more than 6 months and during the study received beta-Epo twice weekly, at the end of the first and third hemodialysis in the week. The dose of beta-Epo was adjusted to attain the hemoglobin target range defined by European best practice guidelines for the management of anemia in patients with chronic renal failure17. In addition, iron status was assessed and supplementary iron given according to the same guidelines.
The last beta-Epo dose prior to pharmacokinetics blood sampling was omitted in all patients but recontinued at the first hemodialysis session after the study with the dose used before. For pharmacokinetic assessment 75U/kg of beta-Epo was administered subcutaneously at the end of the first hemodialysis in the week. Venous blood was collected into heparinized tubes before and at 2, 6, 8, 12, 24, 48, and 72 (only in five patients) h after beta-Epo administration. Blood samples were centrifuged for 10 min and plasma stored frozen until analysis. Erythropoietin (Epo) concentration was determined in all samples on the same day by chemiluminescent immunoassay for erythropoietin on an Immulite 2000 analyzer (Siemens Healthcare Diagnostics).
Pharmacokinetics
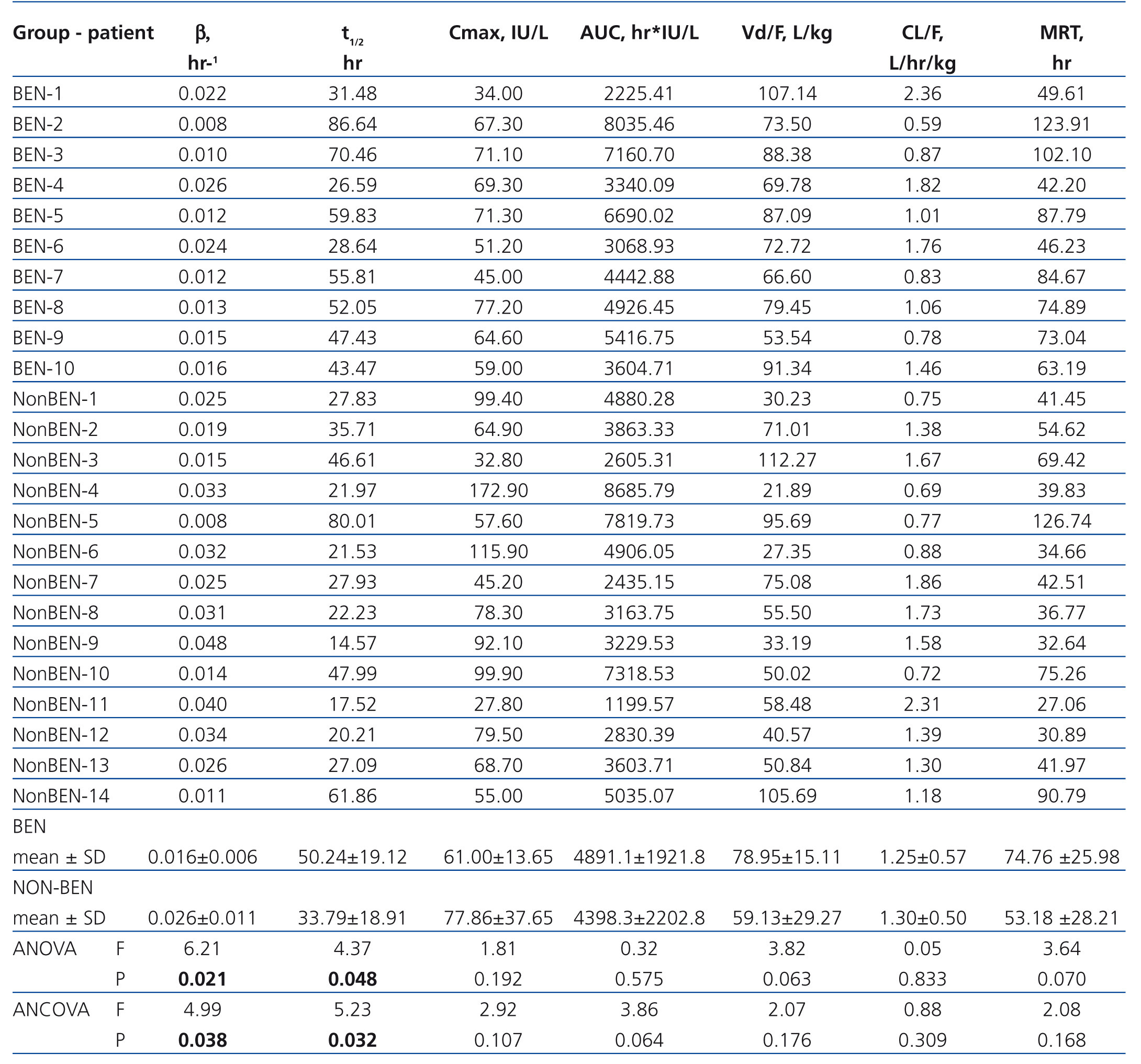
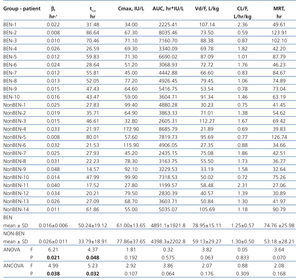
For pharmacokinetic analysis the predose plasma level of Epo was subtracted from all postdose levels of Epo for each patient. Noncompartmental pharmacokinetic analysis using Kinetica software (Thermo Scientific, ver. 5.0) was performed in order to calculate following pharmacokinetic parameters: terminal elimination rate constant (β), half-life of elimination (t1/2), maximum concentration (Cmax), time of reaching Cmax (tmax), area under the curve from time of dosing extrapolated to infinity (AUC), apparent volume of distribution (Vd/F), apparent clearance (CL/F), mean residence time (MRT).
Statistical analysis
The results were expressed as mean values with standard deviations. The significance of differences between mean values for groups was calculated using the Mann-Whitney U test and Student’s t-test. One-way ANOVA was used to compare pharmacodynamics parameters between the groups (BEN and non-BEN). Analysis of covariance (ANCOVA) was used to adjust these differences for patient characteristics that differed between groups: age, sex, hemodialysis duration, ferritin, PTH as well as ACEI use. All analyses were performed using the SPSS (ver. 20) program.
RESULTS
The study involved 24 hemodialysis patients, 10 patients with BEN and 14 with other kidney diseases, selected from 96 patients (40 BEN and 56 others) who met the inclusion criteria. Their main characteristics are presented in Table 1. Patients with BEN were significantly older and had spent less time on hemodialysis than non-BEN patients. No between group differences were found for gender, mean weight, Kt/V and the proportion of subjects using ACEI. None of the participants used ARA II.
Results of laboratory analyses in the examined individuals are given in Table 2. Mean hemoglobin level for the BEN group was 112±5.5g/L and for the non-BEN group 109±4.4g/L, and mean beta-Epo doses were 87±16.5 and 66.5±28.3U/kg/week (p=.051), respectively. Iron reserves were significantly higher in the BEN than in the non-BEN group. Predialysis serum creatinine and PTH concentrations were significantly lower, and serum calcium concentration was significantly higher in the BEN than in the non-BEN group. Basal plasma Epo concentration in BEN patients ranged between 7.2 and 40.1U/L and in non-BEN patients between 6.7 and 31.2U/L, but the difference between the two groups for mean concentration was not statistically significant (20.1±10.3U/L; vs. 15.1±8.1U/L; p=.1964).
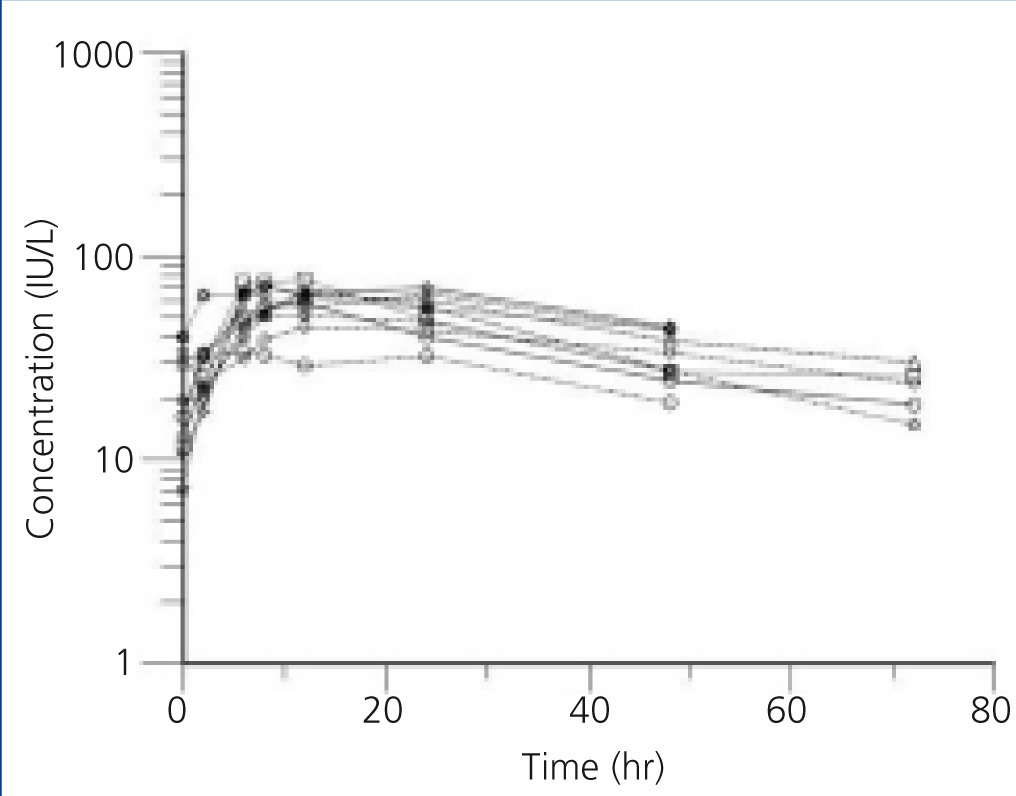
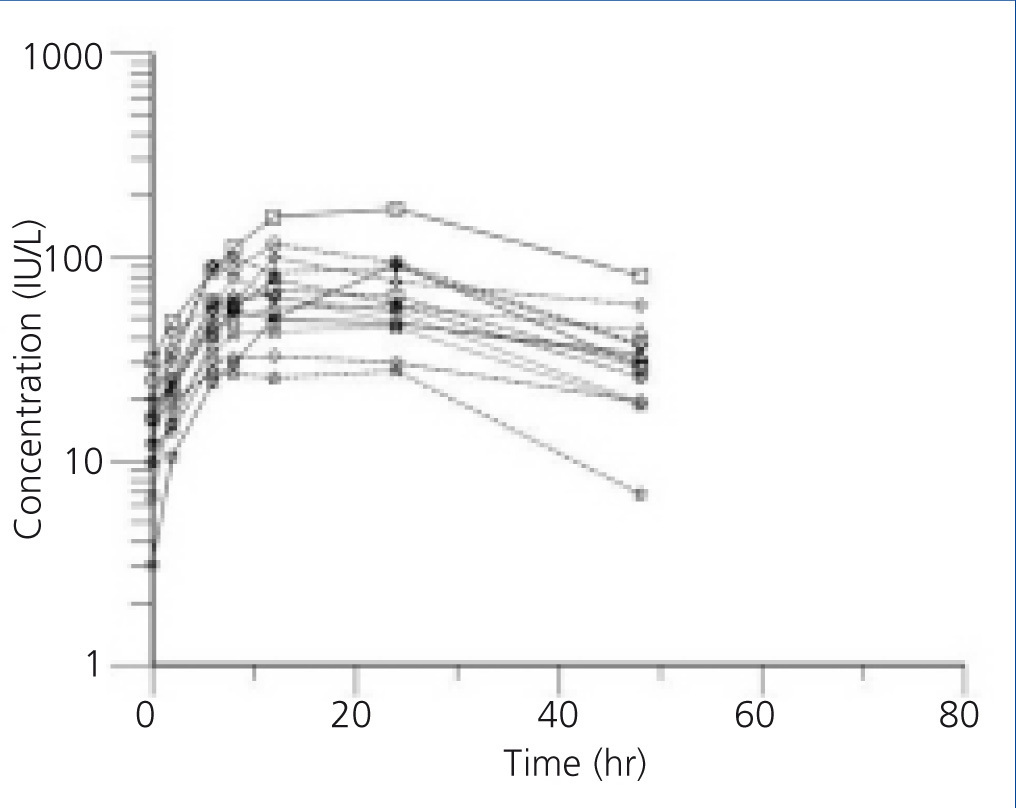
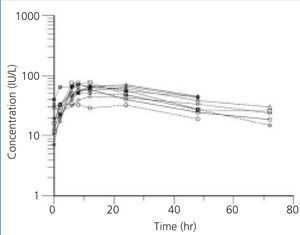
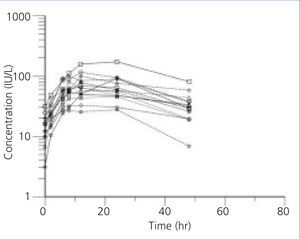
The individual profiles of Epo concentration after subcutaneous administration of 75U/kg of beta-Epo are presented in Figure 1 and Figure 2. After beta-Epo administration plasma Epo slowly increased and Cmax was achieved after 10.6±2.2 h in BEN patients, and after 14.6±6.16 h in non-BEN patients (p=.697). The presented profiles show greater inter-individual variability in Epo concentration but a faster decrease of Epo level during the elimination phase in non-BEN than in BEN patients.
The pharmacokinetic parameters of Epo are presented in Table 3. BEN patients differed markedly from non-BEN patients only in β (0.016±0.006 vs. 0.026±0.011L/h; p=.021) and t1/2 (50.24±19.12 vs. 33.79±18.91 h, p=.048). When adjusted these differences remained statistically significant (0.017±0.011 vs. 0.026±0.009; p=.038 and 57.10±24.54 vs. 33.32±9.12; p=.032 respectively). Also, the difference in other pharmacokinetic parameters presented in table 3 remained insignificant after adjustment.
DISCUSSION
In the present study beta-Epo pharmacokinetics was examined in BEN and non-BEN patients. After subcutaneous administration of 75U/kg of beta-Epo, plasma Epo concentration profiles showed smaller inter-individual variability in BEN than in non-BEN patients. The value of t1/2 was significantly greater in BEN than in non-BEN patients, but the difference in Cmax and tmax was not detected under this study design.
A number of pharmacokinetic studies of ESA have been published, but comparison between them is difficult due to numerous methodological differences. Recent studies were largely devoted to the pharmacokinetics of new ESAs10,12,14 and were usually carried out in healthy persons. The present study was initiated from earlier data involving 146 BEN and 94 non-BEN patients, which showed that significantly higher beta-Epo doses for reaching and maintaining target hemoglobin level were necessary in BEN than in non-BEN patients.8 In the present study the difference in the mean beta-Epo dose between the two groups examined did not reach statistical significance, probably due to the small number of patients involved. A number of factors may contribute to insufficient hematopoietic response to ESAs: iron deficiency most often caused by blood loss, secondary hyperparathyroidism, hemolysis, malignancy, malnutrition, inadequate dialysis, older age, inflammation.18,19 Most of these factors were exclusion criteria for this as well as for our previous study. In addition, none of the mentioned factors for Epo hyporesponsiveness were significantly more pronounced in BEN than in non-BEN patients, except for older age. Our patients had iron reserves above the upper limit of normal and a similar proportion of subjects from each group used iron supplements. Elevated CRP20,21 and PTH21 were found to be associated with delayed or diminished response to ESA treatment. Our groups had similar CRP levels but PTH level was significantly lower in BEN patients than in the others. ACEI and ARA II can increase the need for ESA18,22 but no significant difference was found between BEN and non-BEN patients in the relative number using ACEI (none of them used ARA II). The question arose whether the difference in beta-Epo dose could be caused by different pharmacokinetics of Epo in BEN and non-BEN patients.
After subcutaneous administration of beta-Epo the mean Cmax of Epo in the two groups receiving the same dose did not differ significantly, but inter-individual differences in this concentration were higher in non-BEN (coefficient of variation, CV=48.4%) than in BEN patients (CV=22.4%). Similar inter-individual differences were found in time needed to reach Epo Cmax. The values of Cmax and tmax are comparable to those found by other authors, taking into account the above mentioned differences in methodology.11,16,23,24 Higher inter-individual variability in non-BEN compared to BEN patients might be due to differences in group composition: the BEN group was more homogenous, consisting of patients with a single disease, while the non-BEN group included patients with diverse kidney diseases.
On the other hand, elimination of Epo was slower in BEN than in non-BEN patients. The value of t1/2 obtained in non-BEN patients was comparable to that found elsewhere for hemodialysis patients11 and healthy individuals.25 This indicates that BEN patients were characterized by slower Epo elimination, and therefore with prolonged MRT as well. MRT above 60 hr had 7/10 BEN patients, but 4/14 non-BEN. Vd as primary pharmacokinetic parameter, for conventional drugs, affects the value of t1/2 in a proportional manner and not vice versa. Our results suggest that BEN patients have tendency of higher Vd values, and significantly higher t1/2 (Table 3). However, for protein and peptide drugs, dependency on active tissue uptake, binding to intra- and extravascular proteins can substantially increase the value of Vd. Therefore, not only the distribution by itself, but also the process of elimination and interaction directly with the Epo receptor on the red blood cell surface play an important role in increasing the values of Vd for Epo in comparison to its physiological distribution limited to extravascular space in the body.
Despite long clinical experience with ESAs, the mechanisms involved in their elimination have not been fully elucidated. It seems most likely that both native Epo and recombinant drugs are degraded following receptor-mediated uptake, mainly in the bone marrow.26-29 It might be speculated that slower elimination and greater Vd/F of Epo in BEN patients originated either from lower affinity and slower binding of Epo to its receptors or reduced internalization and/or degradation rate. In addition, recently described mechanisms independent of the Epo receptor elimination pathway,29 may also contribute to slower Epo elimination in BEN patients.
The main limitation of our study was the small number of patients in each group, and they were not matched in age. BEN is a slow progressive chronic kidney disease and patients who progress to end-stage renal disease usually start hemodialysis in the seventh decade of life.30 Previous studies of Epo pharmacokinetics identified age and body weight as covariates influencing absorption and elimination of Epo.31-33 In a study of beta-Epo pharmacokinetics in healthy adult males Hayashi et al.31 detected a significant negative correlation between the elimination rate constant and age and creatinine. As the main organs involved in Epo elimination are the kidneys and bone marrow and both kidney function and bone marrow cellularity decrease with age, the authors suggested that age and creatinine influence Epo elimination. Although an impact of age on Epo elimination in our patients cannot be fully excluded, neither age nor other covariates that differed between the groups was found affect Epo elimination in the ANCOVA model. On the other hand, residual renal function was maintained longer in BEN patients than in those with other kidney diseases34 and our BEN patients were shorter on hemodialysis treatment than non-BEN patients. Therefore, renal clearance of Epo could be higher in BEN than in non-BEN patients. As previous studies showed that the kidneys contribute to Epo elimination in a very minor fashion35 and residual kidney function in patients more than three years on dialysis is most often negligible, it is not expected that renal elimination of Epo could be of any influence.
CONCLUSION
Pharmacokinetic analysis of beta-erythropoietin found a significantly longer t1/2 in BEN than in non-BEN patients indicating that treatment with ESA could have different effects in different diseases. These findings need to be confirmed in a well-controlled study with a larger sample size in order to establish population pharmacokinetics of beta-Epo in BEN patients, to evaluate the effects of physiopathological factors on the disposition kinetics of beta-Epo and to find potential predictive factors for dosage individualization.
Acknowledgments
This work was conducted as a part of projects No 175089 and 175023 funded by the Ministry of Science, Education and Technological Development, Belgrade, Republic of Serbia.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Main characteristics of the patients
Table 2. Results of laboratory analyses in examined patients
Table 3. Individual and mean (± SD) values of pharmacokinetic parameters of rHuEpo after subcutaneous administration of 75 U/kg to examined patients
Figure 1. Plasma erythropoietin concentration profiles in BEN patients after subcutaneous administration of 75 U/kg of beta-erythropoietin
Figure 2. Plasma erythropoietin concentration profiles in non-BEN patients after subcutaneous administration of 75U/kg of beta-erythropoietin