Introducción: Las concentraciones séricas de fósforo muestran una gran variabilidad en los pacientes con enfermedad renal crónica avanzada (ERCA) no en diálisis. El tratamiento con diuréticos puede influir en la severidad de las alteraciones óseo-minerales relacionadas con la ERCA, pero su efecto sobre los niveles de fósforo sérico es menos conocido. Objetivos: Determinar si existe una asociación independiente entre los niveles de fósforo sérico y el tratamiento con diuréticos, e investigar los mecanismos por los que los diuréticos podrían afectar el metabolismo del fósforo. Material y métodos: Estudio transversal en el que fueron incluidos 429 pacientes con ERCA. Además de las determinaciones analíticas convencionales, se incluyeron los siguientes parámetros: excreción urinaria de fósforo en 24 horas, reabsorción tubular máxima de fósforo (TmP) y fracción de excreción de fósforo (FEP). Resultados: El 55 % de los pacientes estaba en tratamiento con diuréticos. Con respecto a los no tratados con diuréticos, los que recibieron este tratamiento mostraron una concentración media de fósforo sérico significativamente superior (4,78 ± 1,23 vs. 4,24 ± 1,04 mg/dl; p < 0,0001), así como una mayor TmP (2,77 ± 0,72 vs. 2,43 ± 0,78 mg/dl; p < 0,0001). Por regresión lineal y logística múltiple, las asociaciones entre diuréticos y concentraciones de fósforo sérico o hiperfosfatemia (fósforo sérico > 4,5 mg/dl) mantuvieron las significaciones estadísticas tras ajuste con las principales variables confundentes. En los pacientes con la máxima carga de fósforo ajustada a función renal, aquellos tratados con diuréticos mostraron una FEP significativamente menor que los no tratados con diuréticos. Conclusión: El tratamiento con diuréticos en la ERCA se asocia a concentraciones más elevadas de fósforo sérico. Los diuréticos podrían interferir de forma indirecta con la máxima capacidad compensatoria renal de excretar fósforo. El tratamiento con diuréticos debería ser tenido en cuenta en los estudios que relacionan las concentraciones de fósforo sérico y las alteraciones cardiovasculares.
Background: Serum phosphate concentrations usually show great variability in patients with advanced chronic kidney disease (ACKD) not on dialysis. Diuretics treatment can have an influence over the severity of mineral-bone metabolism alterations related to ACKD, but their effect on serum phosphate levels is less known. Objectives: This study aims to determine whether diuretics are independently associated with serum phosphate levels, and to investigate the mechanisms by which diuretics may affect phosphate metabolism. Material and Method: 429 Caucasian patients with CKD not on dialysis were included in this cross-sectional study. In addition to conventional serum biochemical measures, the following parameters of renal phosphate excretion were assessed: 24-hours urinary phosphate excretion, tubular maximum phosphate reabsorption (TmP), and fractional excretion of phosphate (FEP). Results: 58% of patients were on treatment with diuretics. Patients on diuretics showed significantly higher mean serum phosphate concentration (4.78±1.23 vs. 4.24±1.04mg/dl; P<.0001), and higher TmP per GFR (2.77±0.72 vs. 2.43±0.78mg/dl; P<.0001) than those not treated with diuretics. By multivariate linear and logistic regression, significant associations between diuretics and serum phosphate concentrations or hyperphosphataemia remained after adjustment for potential confounding variables. In patients with the highest phosphate load adjusted to kidney function, those treated with diuretics showed significantly lower FEP than those untreated with diuretics. Conclusions: Treatment with diuretics is associated with increased serum phosphate concentrations in patients with ACKD. Diuretics may indirectly interfere with the maximum renal compensatory capacity to excrete phosphate. Diuretics should be considered in the studies linking the relationship between serum phosphate concentrations and cardiovascular alterations in patients with CKD.
INTRODUCTION
The change in phosphate metabolism is an invariable consequence of chronic kidney disease (CKD). As the glomerular filtration rate (GFR) decreases, there is a requirement for a compensatory increase in fractional excretion of phosphate, in order to maintain serum phosphate levels within the normal ranges. The dietary phosphate load, parathyroid hormone (PTH), and phosphatonins (fibroblast growth factor (FGF) 23, FGF-7, secreted frizzled-related protein 4, etc.) regulate renal excretion of phosphate by adjusting the expression of sodium-phosphate transporters (NaPi-IIa, NaPi-IIc, and type III PiT-2) in the apical membrane of proximal tubule cells.1,2 This compensation mechanism is usually effective until the more advanced stages of renal failure. However, serum phosphate concentrations show great variability in patients with stages 3-5 predialysis CKD.3,4 In addition to the severity of renal failure and the phosphate load, other factors may also favour the development of hyperphosphataemia, causing interference in the compensation mechanisms of renal excretion of phosphate.
Diuretics are used very frequently in CKD; these drugs may affect mineral metabolism, this being one of several adverse effects.5,6 Furosemide increases renal calcium excretion and may aggravate secondary hyperparathyroidism in patients with CKD.5,6 By contrast, thiazides reduce calciuria, although this effect appears to have very little impact on PTH levels.6,7 However, there have been few studies on the effect of diuretics on phosphate metabolism in CKD.
In a previous study, we observed a higher prevalence of hyperphosphataemia in CKD patients treated with diuretics.8 Another recent study also shows that moderate CKD patients treated with diuretics present significantly higher phosphate levels than patients not treated with diuretics.6 Although this association appears to be independent of a number of potential confounding factors,8 causality is still uncertain.
The objectives of this study were to confirm whether diuretics are independently associated with higher levels of serum phosphate in advanced predialysis CKD, and to investigate the mechanisms by which diuretics may affect phosphate metabolism.
MATERIAL AND METHOD
This cross-sectional study included 429 Caucasian patients (mean age 67±14 years, 201 were women). All patients were recruited consecutively in the Advanced Chronic Kidney Disease consultation during the period between June 2008 and December 2011 with the following inclusion criteria: age over 18, GFR under 40ml/min/1.73m2, not having begun dialysis or being a kidney transplant patient, absence of acute intercurrent illness and severe changes in nutritional status. On extraction of samples for the study, no patient was being treated with phosphate binders or vitamin D.
Exclusion criteria were: treatment with corticosteroids and/or calcineurin inhibitors and patients with paraproteinaemia or multiple myeloma.
The information on the treatment that the patients were receiving was obtained by anamnesis and a review of medical records.
Laboratory methods
All samples for biochemical analysis were obtained from peripheral venous blood after an overnight fast. Patients were requested to bring the urine collected in the previous 24 hours on the day of sample extraction. The concentrations of phosphate, calcium, urea, creatinine and proteins in blood and urine were measured by conventional methods (Advia® Chemistry, Siemens Healthcare Diagnostics). The bicarbonate and plasma ionised calcium concentrations were measured by gasometry (ABL800 FLEX, Radiometer Ibérica). Also included in the study were serum albumin, uric acid, magnesium (colorimetric xylidyl blue) determinations and PTH levels (1-84 molecule, a chemiluminescent immunoassay, Diasorin).
Creatinine and urea clearances were measured and GFR was estimated as half the sum of these two clearances. GFR was also estimated by the MDRD equation with standardised creatinine values.9
The indirect estimation of protein intake was determined with the protein equivalent of non-protein nitrogen appearance (PNPNA), calculated by the combined Cottini et al. and Maroni et al. formulas, as described by Bergström et al.10
Phosphate excretion was calculated in the 24-hour urine samples and was presented as total and normalised excretion to the GFR measured (milligrams of phosphate excreted in 24 hours per ml/min/1.73m2 of GFR). The latter parameter aims to estimate the daily phosphate load of the patient normalised to their renal function.
The calcium excretion rate was calculated according the formula: urine calcium x plasma creatinine/urine creatinine
Tubular maximum phosphate reabsorption by GFR was calculated using the following formula: plasma phosphate - (urine phosphate/urine creatinine) x blood creatinine.
Fractional excretion of phosphate, expressed as a percentage, was calculated using the formula: (urine phosphate x plasma creatinine x 100)/(plasma phosphate x urine creatinine).
Study design and statistical methods
Transversal study comparing serum phosphate concentrations and phosphate renal excretion in patients treated or not with diuretics. The independent association between treatment with diuretics and phosphate levels or hyperphosphataemia (serum phosphate>4.5mg/dl) was also analysed by linear and multiple logistic regression.
In order to establish the maximum compensatory renal capacity to excrete phosphate, the fractional excretion of phosphate was correlated with the phosphate load normalised to kidney function.
To estimate the size of the sample, the following assumptions were made: Type I error (alpha) of 0.05; power of the study 80%; clinically significant difference of serum phosphate concentrations between subgroups of 0.40 mg/dl; and standard deviation of serum phosphate concentrations of 1.1mg/dl. Thus, the minimum number of patients that should be included patients was estimated at 424.
To compare continuous variables in patients treated or not with diuretics, the Student t test or the Mann-Whitney test were used, depending on the characteristics of the variable distribution. The χ2 test was used to compare categorical variables between subgroups.
The discrimination power of total phosphate excretion normalised to GFR to associate with hyperphosphataemia was analysed by ROC (receiver operating characteristic) curves.
To establish the independent association of diuretic treatment with serum phosphate levels or hyperphosphataemia, we used multiple linear and logistic regression, respectively. Independent variables included in these models were: age, sex, GFR, phosphate load, diabetes, serum albumin, serum bicarbonate, proteinuria, PTH and estimated protein intake (PNPNA). For the selection of covariates with better predictive models, the automatic process of conditional progressive elimination was used (backward).
Data were presented as mean ± standard deviation. A p less than .05 was considered as statistically significant. For statistical analysis and producing graphs, the SPSS software version 15.0 (SPSS, Chicago, USA) and STATA version 11.1 (Stata Corporation, Texas, USA) were used.
RESULTS
Differences between patients with and without diuretics
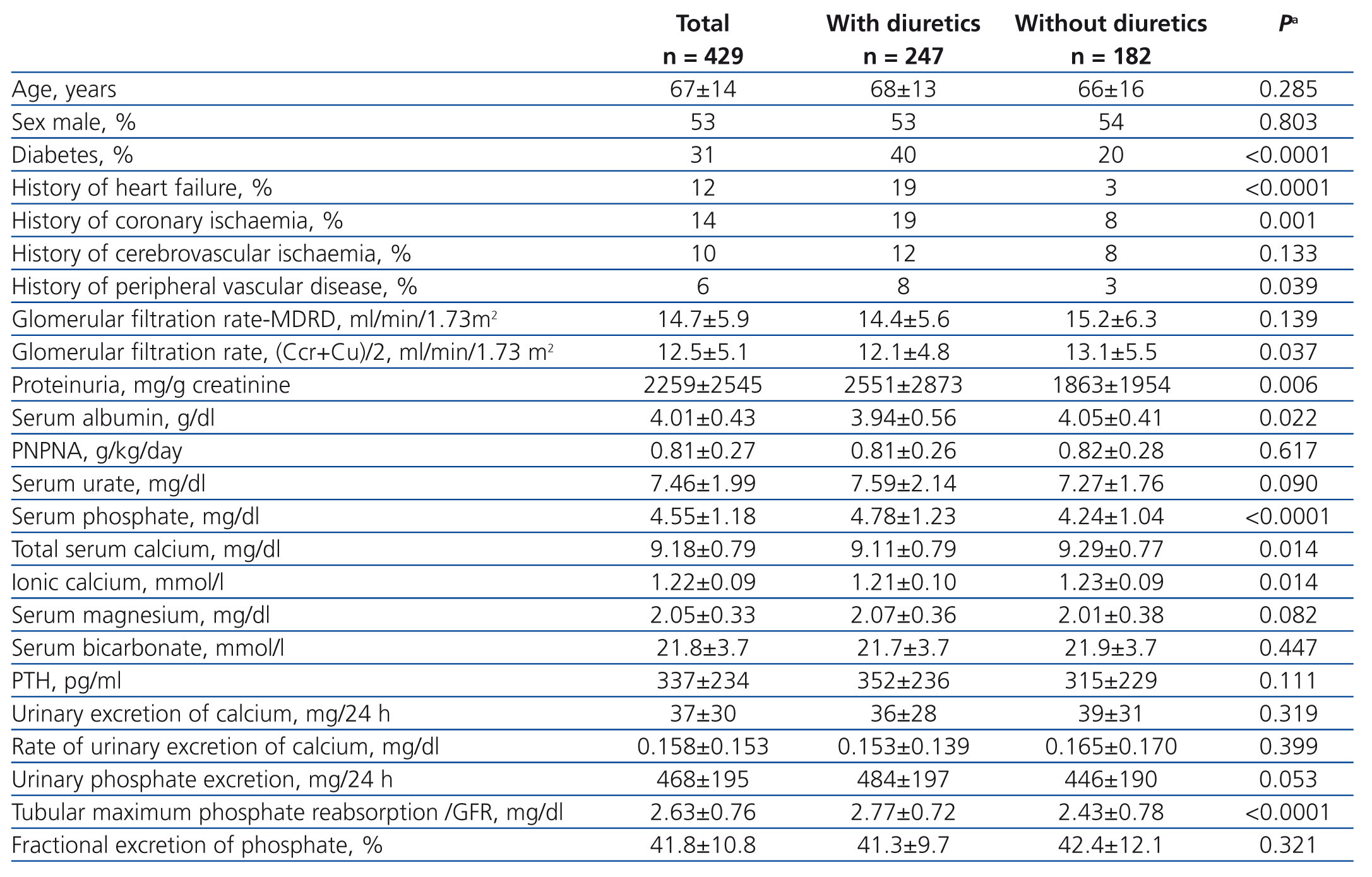
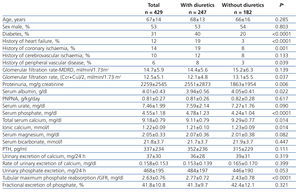
The percentage of patients treated with diuretics was 58%. Table 1 shows the demographic, clinical and biochemical characteristics of the total group and the two subgroups according to whether or not they were treated with diuretics.
A higher percentage of patients treated with diuretics had diabetes mellitus, a history of heart failure, ischaemic heart disease and peripheral ischemia. Patients with diuretics showed higher proteinuria and lower concentrations of serum albumin than those not treated with diuretics. There were no significant differences regarding age, sex or GFR.
Patients treated with diuretics showed a mean concentration of serum phosphate significantly higher than those not treated with diuretics (4.78±1.23 vs. 4.24±1.04 mg/dl, 95% confidence interval of the difference = 0.76 and 0.32mg/dl, p<.0001).
Tubular maximum phosphate reabsorption was also significantly higher in patients treated with diuretics, although the total urine phosphate excretion and fractional excretion of phosphate showed no significant differences between subgroups treated and those not treated with diuretics (Table 1).
Patients treated with diuretics showed lower levels of total and ionised calcium, although the differences in the PTH and urinary calcium excretion levels were not significant (Table 1).
The type of diuretic most used was furosemide (151 patients), followed by torasemide (68 patients) and thiazides (23 patients). Serum phosphate levels in each subgroup by type of diuretic were significantly higher than those in the subgroup without diuretics: the furosemide subgroup (serum phosphate 4.80±1.28mg/dl, p=.001); the torasemide subgroup (serum phosphate = 4.72±1.19mg/dl, p<.05), and the thiazides subgroup (serum phosphate = 4.96±1.11mg/dl, p<.05).
The tubular maximum phosphate reabsorption was also significantly higher in each of the subgroups treated with diuretics with respect to those not treated. No significant differences were found between subgroups in the rest of the parameters studied.
Determinants of serum phosphate levels and hyperphosphataemia
Hyperphosphataemia (serum phosphate> 4.5mg/dl) was observed in 183 patients (43%).
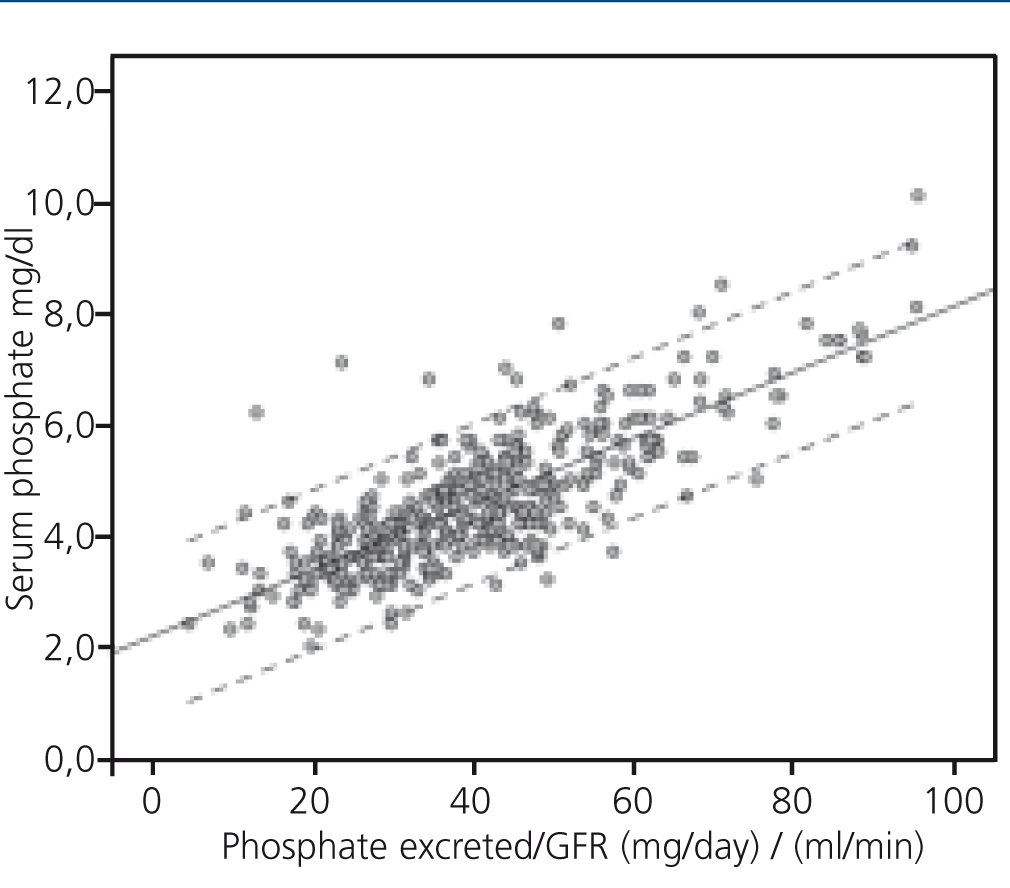
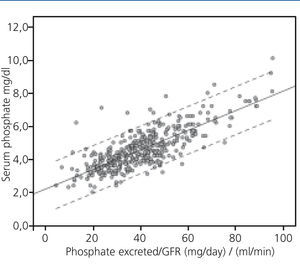
In univariate linear regression analysis, serum phosphate levels were correlated with GFR (R2=0.360, p<.0001) and total urinary excretion of phosphate (R2=0.040, p<.0001). However, the interaction of these two parameters, that is, the load of phosphate normalised to GFR, substantially improved the correlation with serum phosphate concentrations (R2=0.620) (Figure 1).
Serum phosphate levels were also correlated positively with proteinuria (R2=0.126, p<.0001) and negatively with serum albumin concentrations (R2=0.034, p<.0001).
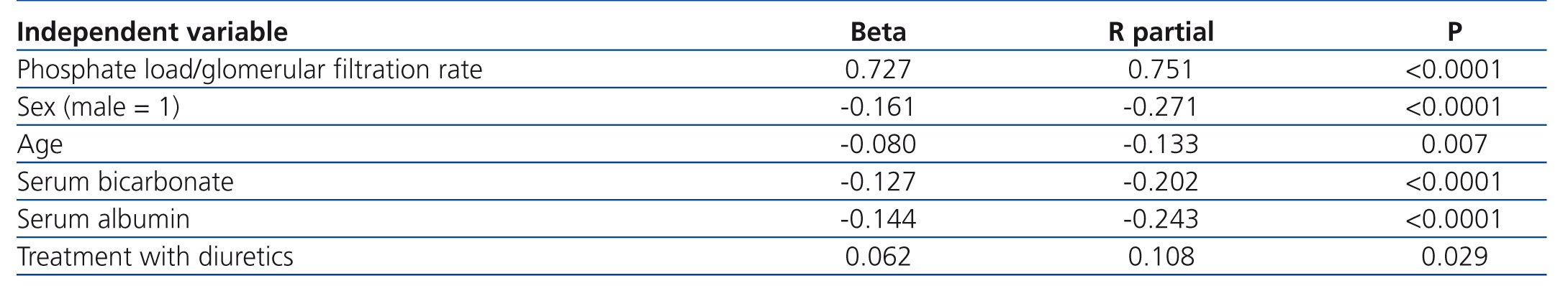
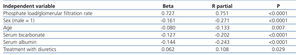
Using multiple linear regression (Table 2), the phosphate load normalized to the GFR was the best determining factor of levels of serum phosphate (beta=0.721), followed by age, sex, serum albumin, serum bicarbonate and treatment with diuretics.
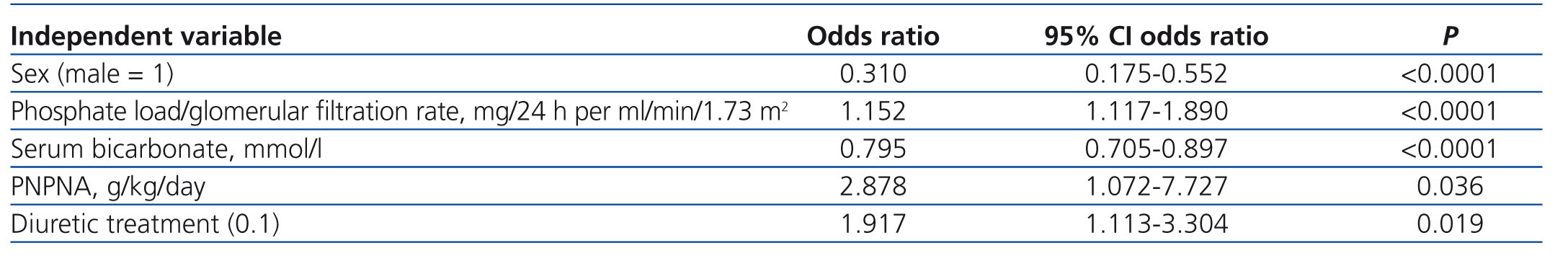
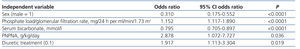
By multiple logistic regression (Table 3), treatment with diuretics was also independently associated with hyperphosphataemia (odds ratio=1.917, p=.019).
In the ROC curve analysis, the phosphate load normalised to GFR was significantly associated with hyperphosphataemia (area under the curve=.861, p<.0001). A value of 40mg of daily urinary excretion per ml/min/1.73 m2 of GFR marked the cut-off point for hyperphosphataemia, with a sensitivity and specificity, both of 75%.
Differences in fractional excretion of phosphate in patients treated and not treated with diuretics
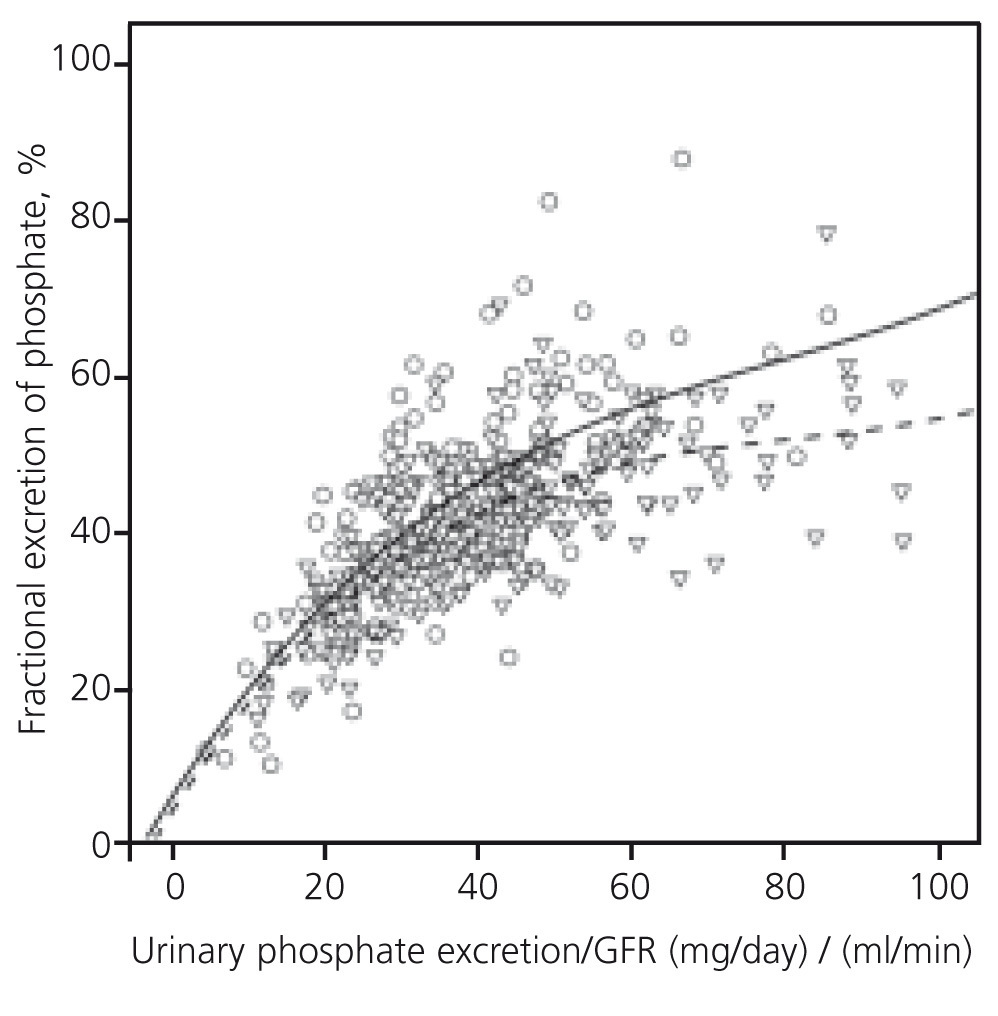
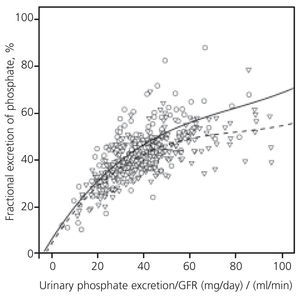
Figure 2 shows curves that best fit the correlation between fractional excretion of the phosphate and GFR-normalised phosphate load in both subgroups. The two curves were virtually identical in the lower section of phosphate load. However, in patients with phosphate overload, the maximum compensatory capacity to excrete phosphate, represented by the maximum fractional excretion of phosphate, reached higher levels in patients not treated with diuretics.
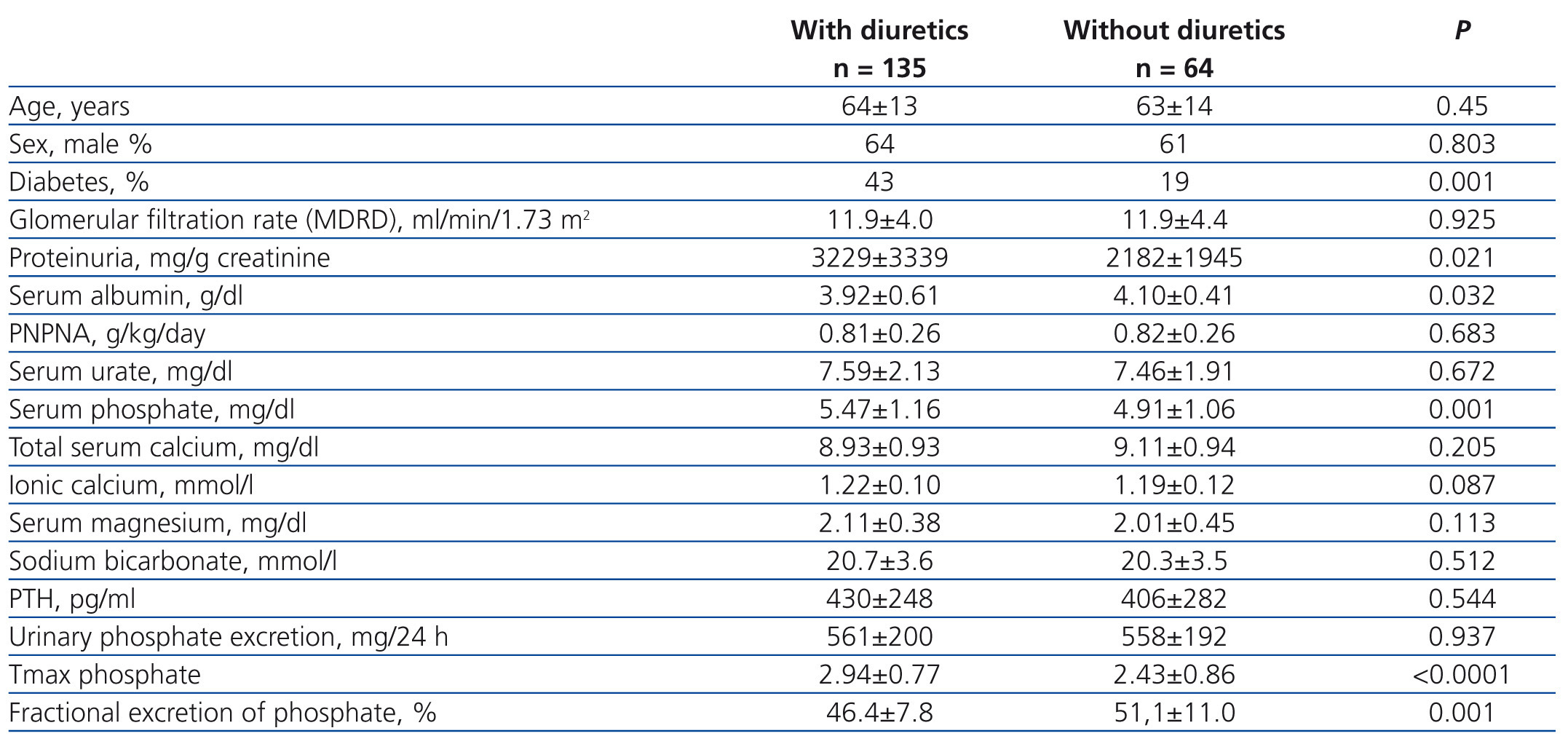
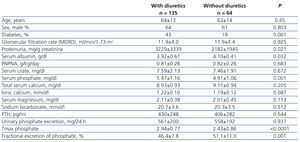
A separate analysis of the data for patients with higher phosphate load normalised to GFR (Table 4) showed that both serum phosphate levels and maximum tubular reabsorption of phosphate were significantly higher in patients treated with diuretics, while the fractional excretion of phosphate was significantly higher in patients not treated with diuretics.
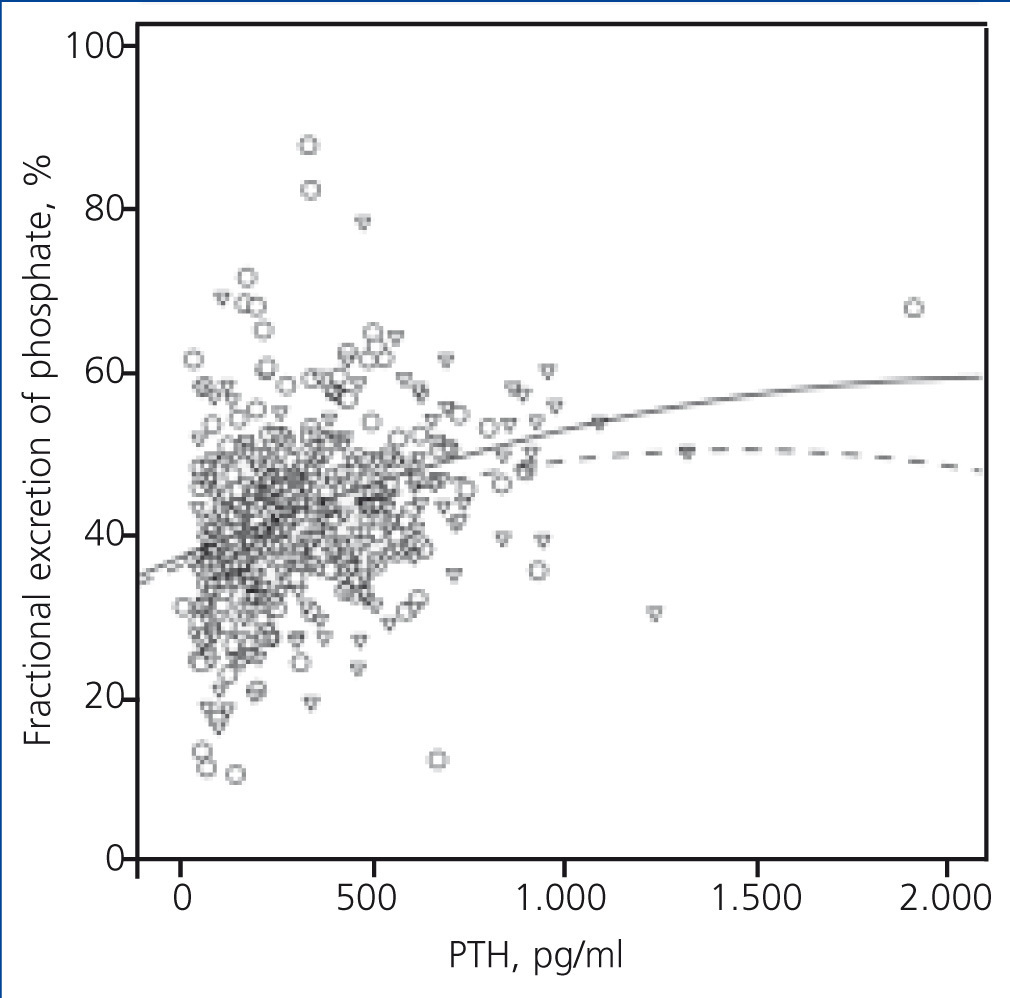
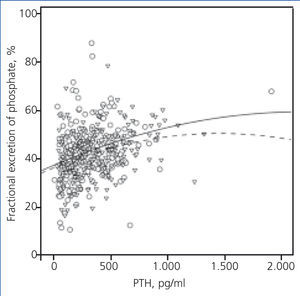
The expected correlation between PTH levels and fractional excretion of phosphate also showed differences in accordance with diuretic treatment (Figure 3).
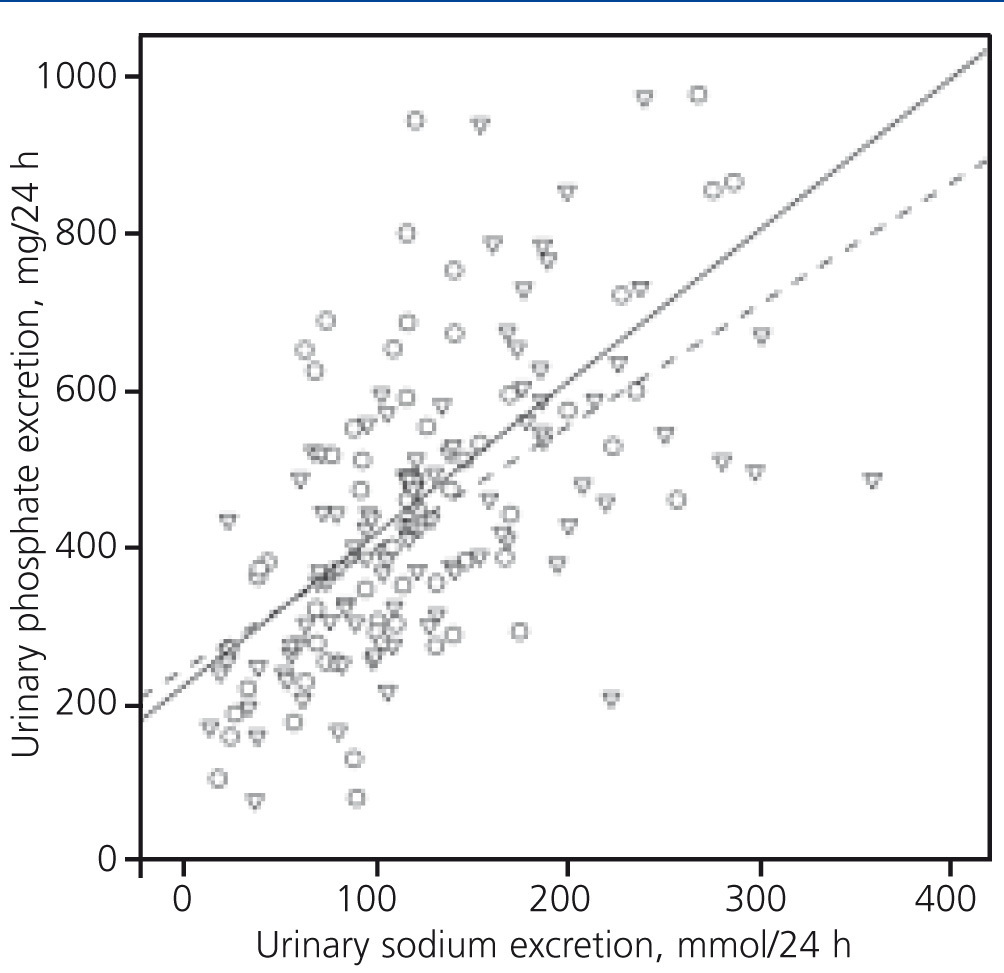
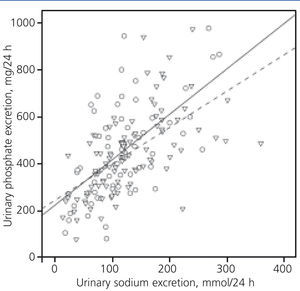
The association between the excretion of phosphate and sodium in 24 hr. urine in a subgroup of 160 patients in which these two parameters were determined, showed an interesting divergence from natriuresis values of 50mmol/24h (Figure 4). In the same sodium excretion, patients with diuretics excreted less phosphate than those not treated with this drug.
DISCUSSION
The results of this study show that CKD patients treated with diuretics have higher phosphate concentrations than untreated patients. The significant association between diuretic treatment and hyperphosphataemia persisted after adjusting results based on variables that could potentially confound this association. Patients treated with diuretics showed a higher maximum phosphate reabsorption and in situations of higher phosphate load, the patients treated with diuretics showed a fractional excretion of phosphate significantly lower than those untreated.
Although this study cannot prove a causal relationship between the diuretic treatment and high levels of serum phosphate due to the cross-sectional design, these findings suggest that diuretics may directly or indirectly interfere with compensatory mechanisms of renal excretion of phosphate in CKD.
In this study, several parameters are used for renal excretion of phosphate which merit comment for a better understanding of the results. Briefly, the relationship between total urinary phosphate excretion and the measurement of kidney function (GFR), a parameter known as the ‘the phosphate load normalised to GFR’ was the best determining factor of serum phosphate levels in the study population. Urinary excretion of phosphate above 40mg per ml/min/1.73 m2 of GFR (e.g., 400mg of urinary excretion of phosphate in patients with a GFR of 10ml/min or 800mg in patients with 20ml/min of GFR) was the best determining factor of hyperphosphataemia. These findings fully correspond to the pathophysiology of CKD mineral alterations.
The compensatory renal excretion of phosphate, represented as a fraction of phosphate excretion, increases almost linearly as the phosphate load normalised to GFR increases, but up to a maximum level that corresponds approximately to the cut-off point where hyperphosphataemia begins to be observed. This fractional maximum excretion of phosphate was significantly higher in patients not treated with diuretics than in those treated with this medication. This finding may help explain the differences in serum phosphate levels between subgroups.
Diuretics may affect mineral metabolism.5,6 While furosemide increases renal calcium excretion, thiazides increase tubular calcium reabsorption, mainly through a mechanism coupled with tubular sodium reabsorption in response to reduced extracellular fluid volume.11 Thus, the type of diuretic used in CKD patients may influence the calcium balance and the severity of secondary hyperparathyroidism.5,6 However, there have been few studies on the effect of diuretics on serum phosphate levels in patients with CKD.
A direct inhibitory effect of a diuretic on phosphate reabsorption in the proximal tubule has only been described with acetazolamide, a carbonic anhydrase inhibitor.12 The effect of other diuretics on phosphate reabsorption in the proximal tubule largely correlates with its ability as a carbonic anhydrase inhibitor.12 However, there has been no stimulant pharmacological effect of diuretics on tubular transport of phosphate, and therefore, it seems very unlikely that a direct effect of diuretics on phosphate metabolism can explain our findings.
Thiazides have been successfully employed to increase levels of serum phosphate in hypophosphatemic rickets,13 a disease characterised by urinary phosphate loss. The basis for the success of this treatment seems to be related to changes in extracellular volume. The expansion of extracellular fluid decreases tubular phosphate reabsorption.14 By contrast, extracellular volume contraction induced by thiazides may increase tubular phosphate reabsorption.1,2,13 Although in this study we did not measure extracellular volume of patients, some possible mechanisms to explain these findings could be related to the reduction of extracellular volume or effective circulating volume, coupled with changes in tubular sodium and phosphate reabsorption.
Each of the three types of diuretics (furosemide, thiazides and torasemide) used in this study were associated with a significantly higher mean phosphate concentration than that of patients not treated with diuretics. This finding suggests the absence of pharmacological specificity in the hyperphosphataemic effect of diuretics.
In the present study, serum phosphate concentrations correlated positively with proteinuria and negatively with serum albumin. In the multiple linear regression analysis, serum albumin and diuretic treatment maintained significance as predictive variables of phosphate concentrations, but not proteinuria. Other studies have also found this interesting relationship between proteinuria and phosphate levels in blood,15-18 although none provide information on the use of diuretics.
Phosphate has been recognised as a new cardiovascular risk factor and in CKD patients not on dialysis, hyperphosphataemia is associated with increased mortality.4,19 Since the use of diuretics is associated most often with a high cardiovascular risk profile, and while these drugs may increase the levels of serum phosphate, the inclusion of diuretic treatment as a potential confounding factor may help better define the role of phosphate and perhaps of phosphatonins, in the development of cardiovascular complications in the general population and especially in CKD.
This study has several limitations. The cross-sectional design prevents causality and temporality of this association from being established unequivocally. This study was carried out at one single centre, the participants were all Caucasian and most were elderly. Levels of 25-hydroxy-vitamin D, 1,25-dihydroxyvitamin D, FGF-23 or other phosphatonins were not measured. Eight patients (six of them not treated with diuretics) showed a very high fractional excretion of phosphate (>65%), probably related to proximal tubular dysfunction. However, exclusion of these patients did not substantially change the results or the statistical significance of the differences found between patients treated and not treated with diuretics.
In conclusion, treatment with diuretics in advanced CKD is associated with higher serum phosphate concentrations. Diuretics may interfere indirectly with the maximum compensatory capacity of the kidney to excrete phosphate. Diuretics treatment should be considered in studies linking serum phosphate concentrations and cardiovascular changes.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Demographic, clinical and biochemical characteristics of the total study group and the subgroups according to treatment or no treatment with diuretics
Table 2. Multiple linear regression on determining factors of serum phosphate concentrations
Table 3. Multiple logistic regression on determining factors of hyperphosphataemia (serum phosphate > 4.5mg/dl)
Table 4. Clinical and biochemical characteristics of the patients with increased levels of phosphate load adjusted to the glomerular filtration rate (> 40mg/day per ml/min/1.73m2)
Figure 1. Correlation between levels of serum phosphate and phosphate load normalised to the glomerular filtration rate
Figure 2. Correlation between fractional excretion of phosphate and phosphate load normalised to the glomerular filtration rate
Figure 3. Correlation between fractional excretion of phosphate and serum concentrations of the parathyroid hormone
Figure 4. Correlation between urinary phosphate and sodium excretion over 24 hours