Introducción: El síndrome de secreción inadecuada de la hormona antidiurética (SIADH) es la causa más frecuente de hiponatremia en el paciente hospitalizado. Sin embargo, faltan protocolos y algoritmos concretos que faciliten su abordaje terapéutico. Nuestro objetivo fue el desarrollo de dos algoritmos de tratamiento de la hiponatremia secundaria al SIADH en el paciente ingresado. Material y método: Un grupo multidisciplinar español compuesto por 2 especialistas en Endocrinología, 1 en Farmacia Hospitalaria, 2 en Medicina Interna y 2 en Nefrología se reunieron durante un año, bajo la tutela del grupo español del European Hyponatremia Network, y de las respectivas sociedades científicas españolas. Las pautas terapéuticas propuestas fueron basadas en recomendaciones ampliamente aceptadas, la práctica de expertos, guías de consenso, así como en la experiencia clínica de los autores. Resultados: Se elaboraron dos algoritmos de tratamiento. El Algoritmo 1 se dirige al tratamiento de la hiponatremia aguda como urgencia médica de abordaje inmediato, y es de aplicación al tratamiento de la hiponatremia grave tanto de tipo euvolémico como hipovolémico. Se basa en el uso de sueros salinos hipertónicos al 3 % i.v., con pautas de infusión y monitorización. Se expone cómo evitar la hipercorrección de la natremia y cómo corregirla en su caso. El Algoritmo 2 aborda el tratamiento de la hiponatremia no aguda leve o moderada asociada al SIADH. Expone cómo y cuándo usar la restricción hídrica, solutos, furosemida y tolvaptán, para alcanzar eunatremia en el paciente con SIADH. Conclusiones: Se han elaborado dos estrategias complementarias para el tratamiento de la hiponatremia inducida por SIADH, en un intento de fomentar la toma de conciencia acerca de esa patología, simplificar su abordaje y su tratamiento y, así, mejorar su pronóstico.
Introduction: The syndrome of inappropriate antidiuretic hormone secretion (SIADH) is the most frequent cause of hyponatremia in a hospital setting. However, detailed protocols and algorithms for its management are lacking. Our objective was to develop 2 consensus algorithms for the therapy of hyponatremia due to SIADH in hospitalized patients. Material and methods: A multidisciplinary group made up of 2 endocrinologists, 2 nephrologists, 2 internists, and one hospital pharmacist held meetings over the period of a year. The group worked under the auspices of the European Hyponatremia Network and the corresponding Spanish medical societies. Therapeutic proposals were based on widely-accepted recommendations, expert opinion and consensus guidelines, as well as on the authors’ personal experience. Results: Two algorithms were developed. Algorithm 1 addresses acute correction of hyponatremia posing as a medical emergency, and is applicable to both severe euvolemic and hypovolemic hyponatremia. The mainstay of this algorithm is the iv use of 3% hypertonic saline solution. Specific infusion rates are proposed, as are steps to avoid or reverse overcorrection of serum sodium levels. Algorithm 2 is directed to the therapy of SIADH-induced mild or moderate, non-acute hyponatremia. It addresses when and how to use fluid restriction, solute, furosemide, and tolvaptan to achieve eunatremia in patients with SIADH. Conclusions: Two complementary strategies were elaborated to treat SIADH-induced hyponatremia in an attempt to increase awareness of its importance, simplify its therapy, and improve prognosis.
* In agreement with the authors and the editors, this paper has been published also in Medicina Clinica:
Med Clin (Barc) 2013;141(11):507.e1-507.e10. http://dx.doi.org/10.1016/j.medcli.2013.09.002
Introduction
The Syndrome of Inappropriate Antidiuretic Hormone Secretion (SIADH) is characterized by the presence of hypotonic hyponatremia in a context of inadequately diluted urine given the hypoosmolality in plasma, in the absence of a low effective circulating volume. (either with hypovolemia or hypervolemia). For its diagnosis, the following conditions must be excluded: hypotension, renal failure, adrenal insufficiency, severe hypothyroidism, as well as non-osmotic physiologic stimuli of arginine vasopressin (AVP) secretion. In SIADH, a lowering of plasma osmolality (Posm) does not inhibit AVP secretion as expected, and free water is inappropriately reabsorbed at the level of the collecting duct of the nephron, thus inducing further hemodilution and hyponatremia, concomitantly with an inappropriately elevated urine osmolality (Uosm)1,2. In the less frequent Syndrome of Inappropriate Antidiuresis (SIAD), ADH secretion is inhibited, but an activating mutation of collecting duct V2 receptors imitates excessive ADH-induced water reabsorption3-5.
Major advances have taken place over the last few years in our understanding of the negative consequences of hyponatremia. Mild, chronic hyponatremia is not asymptomatic. On the contrary, it is accompanied by mental symptoms, impaired gait, falls6, fractures7-9, and possibly osteoporosis10, as well as increased mortality9, 11-14. Severe hyponatremia per se can be life-threatening, inducing profound cerebral edema and potentially brain herniation and death. Even once hyponatremia is corrected, complete neurologic recovery can take weeks, and some patients will have permanent neurologic sequelae as a consequence of profound hyponatremia15. Yet overly rapid correction of serum sodium can induce osmotic demyelination syndrome (ODS). Adequate and prompt correction of hyponatremia is thus imperative, avoiding and/or dealing immediately with overcorrection, while assuring that eunatremia is ultimately achieved.
Close to 30% of hospitalized patients are hyponatremic at some time during their hospital stay13,16,17, yet hyponatremia is often overlooked and undertreated, even in its most severe forms. A correct diagnosis of the cause of hyponatremia is per se associated with reduced in-hospital mortality, as indicated by a lower death rate in patients with hyponatremia in whom plasma and/or urine Osmolality have been determined18. And specific treatment directed towards correcting hyponatremia is accompanied by a lower death rate.19.
SIADH is considered to be the most frequent cause of hyponatremia in a hospital setting20. However, detailed protocols and algorithms for its management are lacking. A new class of therapeutic agents, the vaptans, is currently available for the treatment of SIADH in the adult. They block the AVP-responsive V2 collecting duct receptors that ultimately induce apical expression of aquaporin 2 channels through which water is reabsorbed following AVP stimulus21. Tolvaptan is the only drug of its class authorized by the European Medicines Agency, and is a selective V2-receptor blocker. Its addition to the therapeutic arsenal of 3% hypertonic saline solution, furosemide, solute, and fluid restriction further warrants a specific guide to the treatment of SIADH-induced hyponatremia.
Our objective was to develop two consensus algorithms for the therapy of hyponatremia due to SIADH in hospitalized patients. These algorithms address acute correction of hyponatremia posing as a medical emergency (Algorithm 1) and correction of mild or moderate, non-acute hyponatremia requiring less aggressive measures (Algorithm 2).
Materials and Methods
The algorithms were developed by a multidisciplinary group made up of 2 Endocrinologists, 2 Nephrologists, 2 Internists, and 1 Hospital Pharmacist. Over the period of a year, the group held meetings and worked on-line, organized under the auspices of the European Hyponatremia Network and the corresponding Spanish Medical Societies.
Given the dearth of evidence in many aspects of the management of hyponatremia, we have based the algorithms we present on widely-accepted recommendations, expert opinion and consensus guidelines22, as well as on the authors’ personal experience. Application of the protocol should be flexible, and adapted to the specific needs of each patient, as is always the case in clinical medicine.
Explanation of the algorithms and their use
The severity of the patient’s neurologic symptoms will be the most important element to be considered when choosing which algorithm to apply initially22, together with other factors presented in tables 1 and 2. Aggressive and prompt correction (Algorithm 1) is particularly important in subjects with acute and/or severe symptomatic hyponatremia indicative of substantial cerebral edema, in patients with hypoxemia23-28, in children and in women of child-bearing age27.
When applying either algorithm, medication that can potentially cause hyponatremia must be discontinued when feasible. Pain and nausea are potent stimuli of AVP secretion and must be adequately treated. If the underlying cause of SIADH-induced hyponatremia is not evident, a Chest x-ray, brain, cervical and thoracic CT, and abdominal ultrasound must be considered. If ordered, these diagnostic tests should follow initial emergency correction of SNa and improvement of patient’s symptoms if hyponatremia is severe.
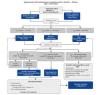
Application of Algorithm 1
In the setting of acute and/or severe hyponatremia with symptoms indicative of profound cerebral edema, the initial aim of this algorithm (figure 1) is reduction of said edema, while avoiding overcorrection of serum sodium. It is applicable to severe euvolemic or hypovolemic hyponatremia per se, regardless of the cause, and thus prior to diagnosis of its origin. The algorithm specifies initial treatment of cerebral edema with 3% hypertonic saline iv, monitoring of the subsequent rise in serum sodium concentration (SNa), and avoidance or treatment of overcorrection of hyponatremia.
Hypertonic saline solution
1. 3% Hypertonic saline is the mainstay of treatment. The mainstay of therapy is the use of 3% hypertonic saline solution (HSS) administered iv29, to assure the movement of water from the intracellular to the intravascular compartment following an osmotic gradient, thereby reducing neuronal edema. A central venous line is unnecessary and can only delay treatment. 3% saline, if unavailable, can be prepared by adding 60 ml of 20% saline to 500 ml of 0.9% isotonic saline. The resulting solution must be shaken before administration. Immediately prior to initiating treatment, blood must be drawn for determination of SNa, serum potassium (SK), urea, creatinine and plasma osmolality (Posm ). Urine for the determination of Na (UNa), K (UK) and Uosm must be obtained as soon as possible, without delaying initiation of hypertonic saline administration. Hypoxemia must be ruled out and corrected if detected, since it worsens prognosis23-28.
Infusion rate. We propose an initial iv infusion rate of 3% HSS at 0.5 ml/Kg/hour or 1-2 ml/Kg /hour depending on the severity of the neurologic symptoms30,31. Patients in coma, or presenting with seizures, stupor or respiratory distress, should be started on an initial infusion rate of 2 ml/Kg/hour In these patients, an alternative can be the administration of an iv bolus of 100 ml of 3% HSS, that can be readministered up to 2 more times at 10-minute intervals, and stopped once the acute symptoms have subsided22,32. We do not advise the use of formulas to estimate the quantity and rate of infusion, since these calculations often lead to overcorrection32-34 and can needlessly complicate therapy.
As previously mentioned, treatment must be particularly aggressive in children and in women of child-bearing age27, given that their risk of severe hyponatremic encephalopathy is higher, potentially leading to subsequent brain herniation and death, or permanent neurologic sequelae. This is also the case for patients with acute hyponatremia. Patients who have recently ingested large quantities of liquid (acute water intoxication) are at special risk, since their neurologic symptoms and serum sodium will often worsen as they continue absorbing water from the gastrointestinal tract35. We note that in these patients, sodium levels in arterial blood are often several mmol/L lower than in peripheral venous blood, and thus the latter can underestimate the true degree of hyponatremia36.
HSS can induce substantial water diuresis, favoring overcorrection. Thus diuresis must be closely monitored to detect frank polyuria (urine output of approximately 2 ml/Kg/hour) and the need to initiate corrective measures directed towards relowering of SNa. These patients are also candidates to receive 1 mcg of sc or iv desmopressin directly upon detection of polyuria, thus preventing an excessive rise in serum sodium32,37.
Specific treatment goals of hypertonic saline infusion. In the treatment of patients with chronic hyponatremia (of a duration of 48 or more hours), the maximum recommended rise in SNa is currently 10 mmol/L in 24 hours, and 18 mmol/L in 48 hours, to avoid the appearance of osmotic demyelination syndrome22. Furthermore, a 4-6 mmol elevation in sodium levels is accompanied by a reduction in cerebral edema of the order of 50%38. A recent study found that patients presenting an initial SNa below 120 mmol/L had a higher mortality rate if their SNa rose less than 6 mmol/L over the first 24 hours of treatment39. Thus, our aim should be to achieve an increase in serum sodium of the order of 6 to 8 mmol over a 24-hour period. Avoiding overcorrection is particularly important in patients at a high risk of developing the osmotic demyelination syndrome such as alcoholics, patients who are malnourished, and those presenting hypokalemia40-42. In these patients maximum correction rates should therefore be from 6 to 8 mmol/L and from 14 to16 mmol/L over 24 and 48 hours respectively. However, in patients with acute hyponatremia SNa can rise more quickly, without negative consequences.
No other treatments that raise serum sodium levels should be given concomitantly with hypertonic saline, with the exception of iv furosemide in patients with a history of heart failure. Since the combination of hypertonic saline and a loop diuretic will increase sodium levels more than the use of hypertonic saline alone, the initial objective in these patients should be a rise in SNa of approximately 4-5 mmol/L with hypertonic saline infusion.
Monitoring and discontinuing hypertonic saline infusion. SNa should be repeated 2 hours after initiating HSS infusion. If sodium levels have risen more than 6 mmol/L, saline infusion must be stopped, since SNa can still rise after the infusion has been discontinued. If serum sodium has increased 1 to 6 mmol/L, infusion can be maintained at the same rate. If there is no increment in SNa , the infusion rate should be increased by 50-100%, taking into account the clinical status of the patient. If bolus therapy has been used, sodium levels should be monitored 30 minutes after the last bolus.
SNa should once more be monitored 2 hours later (4 hours after initiation of saline infusion). If a patient has yet to present an increase of 2 mmol/L, and shows insufficient clinical improvement, the rate of saline infusion should again be increased, with SNa determined once more after two hours. If the patient presents only moderate or mild symptoms, the infusion rate can be maintained and SNa repeated 4-6 hours later. In patients whose SNa has risen over 6 mmol/L , saline infusion should be discontinued. If SNa has risen over 8 mmol/L, corrective measures should be initiated (see below). If the rise in SNa is between 2 and 6 mmol/L, infusion should be maintained, and SNa monitored every 2 hours until it has risen by 6 mmol/L.
One of the most important recommendations we can make in the use of hypertonic saline is that the prescribing physician personally supervise cessation of the iv infusion.
Once hypertonic saline infusion has been discontinued, and until 24 hours have passed since the start of treatment, oral salt can be administered and fluid restriction initiated in patients with SIADH. Other measures that can potentially raise SNa should be avoided, to decrease the risk of overcorrection. We include potassium chloride (KCl) in this category: mild hypokalemia should not be corrected until the following day, since KCl administration will increase serum sodium levels.
Corrective Measures to Relower Serum Sodium
Relowering of serum sodium, following an excessive rise, prevents ODS. The development of ODS has been associated with an excessive rise in serum sodium over a 24 or 48 hour period. ODS can induce death or leave permanent neurologic sequelae. It is for this very reason that the 2007 guidelines22 recommend a maximum serum sodium increment of 10 mmol/L and 18 mmol/L in 24 and 48 hours respectively. However, a rapid reinduction of hyponatremia has been shown to prevent ODS symptoms37,43,44 and has been successfully used to treat patients that have developed ODS45-49.
Specific Measures
If SNa is overcorrected (SNa rise greater than 8 mmol/L following discontinuation of HSS or SNa rise greater than 10 mmol/L 24 hours after initiation of therapy), measures should be taken immediately to once again lower the SNa level. These include oral liquids, 5% iv dextrose solution (4-6 ml/Kg/hour for 2 hours with subsequent reevaluation of SNa) and 1-2 mcg iv or sc desmopressin every 6 hours if and as needed37. When using desmopressin, we recommend a lower initial rate of dextrose infusion: 1.5 to 2 ml/Kg/hour, repeating SNa 2 hours later, and increasing the dextrose infusion rate as necessary. A lowering rate of 1 mmol/L/hour has been recommended50. The association of desmopressin to iv dextrose is particularly important in patients who will not otherwise reabsorb large quantities of water in the collecting duct, as is the case of patients with primary polydypsia, and other patients with low Uosm. With low AVP levels, these patients will have few patent aquaporin-2 channels, (reflected by a low Uosm) and desmopressin should be administered for dextrose infusion to be useful. Iv furosemide can be administered once the desired serum sodium level has been reached, to put a halt to a further reduction in SNa.
Patients at high risk for overcorrection. Certain groups of patients present a higher risk of overcorrection of serum sodium than others, and must be more closely monitored. These include patients in which the cause of hyponatremia is transitory, e.g. hyponatremia-inducing medication which has just been discontinued, or physiologic stimuli of AVP secretion once the stimulus has passed32. Patients with an extremely low salt intake prior to admission also fall into this category.
Patients with adrenal failure, either primary or secondary, are a separate case. They should receive high enough hydrocortisone doses to cover their glucocorticoid and mineralocorticoid needs. SNa can rise very quickly after initiation of steroid therapy, particularly if high doses of hydrocortisone are given, and especially if administered by iv bolus, as when treating an adrenal crisis. In these patients, if SNa rises by 8 mmol/L before 24 hours have elapsed since the start of treatment, 1-2 mcg of iv or sc desmopressin can be administered every 6-8 hours to prevent a further increment in S Na levels. In many patients, however, the infusion of lower doses of hydrocortisone will be sufficient (between 60-150 mg daily51, with or without an intial bolus of 50 mg iv) and will induce a slower rate of correction of SNa levels. In even milder cases, oral hydrocortisone can be given at a dose of 20 mg tid.
Application of Algorithm 2
The aim of algorithm (figure 2) is to achieve eunatremia in patients with SIADH-induced mild or moderate hyponatremia not posing as a medical emergency.
Correction of hypokalemia
If hypokalemia is present, Potassium Chloride supplements should be given, taking care NOT to administer potassium bicarbonate, or potassium supplements that result in the generation of bicarbonate, since the latter will increase renal sodium excretion, and can induce metabolic alkalosis52.
Solute Administration
Patients with SIADH should have an adequate oral salt intake. A hospital diet frequently provides less than 4 grams of sodium chloride a day. Furthermore, many patients eat considerably less than what is provided, and will have an even lower sodium intake. We recommend the association of NaCl supplements, for a minimum consumption of 5 to 8 grams of salt daily.
The administration of large amounts of solute can be used for the treatment of SIADH – induced hyponatremia, acting through the induction of increased renal obligatory water clearance. In this case, oral salt supplementation can range from 3 to 4 grams every 8 hours, and must be even higher in exceptional cases. Close monitoring of blood pressure is essential. Another solute that has been given for the treatment of SIADH hyponatremia is oral urea, at doses of 30 g a day, usually given in two 15 g doses53. Urea could be the therapy of choice in children with SIADH54. However, its low palatability has limited its use.
Fluid Restriction
Currently, fluid restriction is the cornerstone of treatment of SIADH-induced hyponatremia not requiring urgent intervention. By fluid restriction we mean the reduction of all liquids administered to the patient, be it with iv medication, oral liquids, or the intrinsic water content of foods. To adequately initiate fluid restriction, iv medication must be concentrated whenever feasible, and non-essential oral and iv medication discontinued. Oral liquid intake must be limited and foods with a high water content eliminated from the diet (soups, mashed foods, coffee, tea, gelatins, semi-liquid desserts, melons, citric fruits, etc. ).
In our experience, the Furst formula55 is useful in predicting which patients will respond adequately, and is calculated by dividing the sum of UNa and UK (mmol/L) by SNa. Patients with a result less than 0,5 can be limited to 1000 ml of total liquid intake, whereas those between 0,5 and 1 need to be limited to 500 ml of liquid a day. Patients with a result of 1 or more will not respond to fluid restriction, since their nephrons are not capable of eliminating electrolyte-free water. In this case fluid restriction to 500 ml daily is used to prevent worsening of hyponatremia, not to correct it. The response of a specific patient to fluid restriction is not constant, and can vary from one day to another. Therefore, for a correct use of the Furst formula, electrolytes must be measured in serum and urine frequently. We consider a positive response to be an average 2 mmol/L rise in SNa per day over a 2-day period.
Fluid restriction is not practical in all patients, and is simply incompatible with necessary medication or artificial nutrition in others. Another group does not tolerate or does not respond to the required fluid restriction. Furthermore, fluid restriction to 500 ml makes meals less palatable, and can worsen the nutritional status of the patient if used for more than a few days. In these cases, together with those presenting a Furst formula result of 1 or more, alternative therapies must be initiated.
Furosemide Therapy
Furosemide can be useful as a short-term treatment for SIADH hyponatremia when the UOsm is sufficiently high, as it induces an increase in renal free water clearance. For this loop diuretic to be effective, UOsm must be over 350 mOsm/Kg, and preferably over 400 mOsm/Kg. Sodium losses must be compensated by sufficient oral salt intake. Furosemide can be administered iv or orally, in doses ranging from 20 mg iv every 8 to 24 hours, or 40 mg every 8 to 24 hours p.o., although higher doses have been successfully given56,57. The use of this loop diuretic is particularly helpful in patients in whom SIADH will presumably be of limited duration (days), as is the case of patients with pneumonia or drug-induced SIADH when the medication can be discontinued (table 3).
Furosemide can also be useful in patients with serum sodium levels below 120 mmol/L presenting with moderate or mild symptoms, sodium levels higher that 115 mmol/L, and a low risk for herniation (ie the elderly). In this case serum sodium should be repeated 3 to 6 hours after an iv dose.
Tolvaptan Therapy
We recommend the use of tolvaptan, the selective V2 receptor inhibitor58, in patients with SIADH who are not candidates for fluid restriction or furosemide. The drug is particularly useful in patients that will need treatment of their hypotonic hyponatremia for several days, or longer, as occurs in patients with chronic SIADH.
Initiation and monitoring of tolvaptan therapy. We prefer to start tolvaptan in patients in whose Nap has already risen above 119 mmol/L, since patients with lower serum sodium levels seem to be at a higher risk for overcorrection, in our experience as well as in that of others (Dr. Volker Burst, University of Cologne, personal communication).
Tolvaptan therapy must always be initiated while the patient is hospitalized. Care should be taken to assure that during the first day of tolvaptan administration patients not only have free access to liquids, but also are actively encouraged to drink ad libitum, since maximum aquaresis is observed precisely on day 1, when excess circulating water is greatest. Patients who have previously been on fluid restriction are prone to not drinking as much as they desire and must be closely monitored. If a patient has a limited capacity to drink (ie oral or gastrointestinal disease) 5% dextrose solution should be associated from the start. Patients with a nasogastric tube will also need increased liquids. In these cases, diuresis is helpful in estimating increased fluid needs.
The initial recommended dose is of 15 mg p.o., although several groups prefer to start treatment with 7.5 mg59,60, (Dr. Volker Burst, personal communication). It is preferable to administer tolvaptan early in the morning (7 to 9 AM), to facilitate monitoring of the patient’s response, and to assure adequate nocturnal rest. We recommend measuring serum sodium, potassium and POsm as well as sodium, potassium and osmolality in urine at baseline, and 6 hours after the initial dose, with monitoring of fluid intake and diuresis every 6 hours.
Patients whose SNa has not increased by more than 5 mmol/L at 6-hours post-tolvaptan, and are drinking freely can be monitored by fluid intake/diuresis alone, with SNa repeated 24 hours after the initial dose. If at 6 hours from baseline, SNa has risen more than 5 mmol/L, we recommend initiating corrective measures in order to prevent an excessive rise in the 24-hour serum sodium levels, , given that in our experience on day one of tolvaptan therapy SNa will rise at nighttime, when the patient`s fluid intake is low. These measures include an increase in oral fluid intake, and 5% dextrose solution iv infusion, at a rate of 3-4 ml/Kg/hour for 2 hours, followed by a new determination of SNa. We have found the association of desmopressin (iv or sc) to be useful in avoiding overcorrection of SNa in spite of V2 blockade, at doses of 2-4 mcg every 6 hours as needed. When using desmopressin, we recommend starting with a lower rate of dextrose infusion: 2 3ml /Kg/hour for 2 hours, followed by measurement of SNa. The rate of infusion is then increased every 2 hours as necessary to relower sodium levels , with a goal of attaining a maximum increment of 4 mmol/L over baseline. Patients whose SNa has risen more than 5 mmol/L over the first 6 hours should have SNa measured once more 12 hours from baseline. Not all patients drink as needed and thus some present a excessively negative fluid balance. They will also require a new 12 h measurement of SNa.
If 24 hours following the first dose serum sodium levels have increased more than 10 mmol/L, tolvaptan should not be readministered on that day, and corrective measures used as needed, although discontinuation of tolvaptan administration is usually sufficient to relower SNa to desired levels. The drug can be reintroduced on the following day. If SNa has increased by less than 10 mmol/L, a new dose of 15 mg should be administered, and SNa monitored on the following day. When the rise in serum sodium levels is insufficient, the daily dose of tolvaptan can be increased to 30 mg, with a maximum dose of 60 mg/day. Experience with tolvaptan in Europe is still somewhat limited, and its use will surely undergo modifications in the future, particularly regarding doses.
Tolvaptan: General Considerations. Tolvaptan has important drug interactions which must be taken into account before and during its use (Table 4). As a substrate for cytochrome P450 3A4, circulating levels of tolvaptan will increase when used with inhibitors, and will drop when associated with inducers. Tolvaptan at high doses (60 mgday) can increase serum digoxin concentrations.
Tolvaptan should be used as monotherapy for SIADH, and never be administered jointly with hypertonic saline. On day 1 of use, it is preferable not to associate potassium supplements, nor furosemide. Tolvaptan should never be administered to a hypovolemic patient, underlying the importance of a correct diagnosis of SIADH. Tolvaptan is usually well tolerated, and in our experience is very effective for attaining strict eunatremia. However, intense thirst sometimes follows its administration. When accompanied by the ingestion of large quantities of liquid, thirst can lessen the effectiveness of the medication, as can chronic pain. Patients with SIADH type D (NSIAD due to an activating V2 receptor mutation) will probably not respond to tolvaptan treatment61.
Duration of Treatment. Out-patient follow-up. The duration of tolvaptan treatment is determined by the persistence of the underlying cause of the patient’s SIADH (Table 3). When discontinuing tolvaptan, a progressive reduction in dose is always preferable to abrupt withdrawal of the medication. In patients discharged on tolvaptan therapy, we recommend repeating SNa within a week, with new laboratory tests 2 to 4 weeks later, then monthly. In patients with chronic SIADH, in our experience, the tolvaptan dose can be progressively reduced once strict eunatremia is achieved. Patients with particularly low maintenance doses of tolvaptan may need higher doses once more in situations of increased liquid administration, and /or low salt intake, such as typically occurs with hospital readmission.
Conclusions
Hyponatremia is a very common disorder, yet it remains underdiagnosed, underappreciated, and often incorrectly managed. Concrete roadmaps for the treatment of hyponatremic patients are lacking. We have elaborated two complementary strategies to treat SIADH-induced hyponatremia in an attempt to increase awareness of its importance, simplify its therapy, and improve prognosis.
In spite of the frequency and negative repercussions of hyponatremia, there is a paucity of evidence in the field of hyponatremia and its therapy. We are clearly in need of more research directed towards gathering evidence that can optimize therapy.
We hope that the application of this protocol will simplify and help bring consistency to the treatment of SIADH-induced hyponatremia, thus reducing morbidity, and facilitating the accumulation of evidence.
Conflicts of interest
Isabelle Runkle has worked as a consultant for Otsuka and has given sessions sponsored by Otsuka, Amgen and Novartis.
Carles Villabona has worked as a consultant for Otsuka and has given sessions sponsored by Novartis, Ipsen and Otsuka.
Andrés Navarro has worked as a consultant for Otsuka.
Antonio Pose has worked as a consultant for Otsuka, Boehringer, MSD, Rovi and Almirall, and has given sessions sponsored by Otsuka, Almirall, Boehringer, Novartis, Astra, Bristol, Lilly, Bayer, Sanofi, Lacer and Novo.
Francesc Formiga has worked as a consultant for Otsuka and has given sessions sponsored by Otsuka.
Alberto Tejedor has worked as a Nephrology consultant for the Spanish Agency of Medicines and the European Medicines Agency. He has been a consultant for Otsuka and Astra Zeneca.
Esteban Poch has worked as a consultant for Otsuka and has given sessions sponsored by Otsuka.
Table 1. Definitions
Table 2. Classification of hyponatremia according to severity of clinical symptoms
Figure 1. Algorithm 1. Acute therapy
Figure 2. Algorithm 2. Non-acute treatment
Table 3. Probable duration of syndrome of inappropriate antidiuretic hormone secretion treatment as indicated by etiology
Table 4. Tolvaptan drug interactions