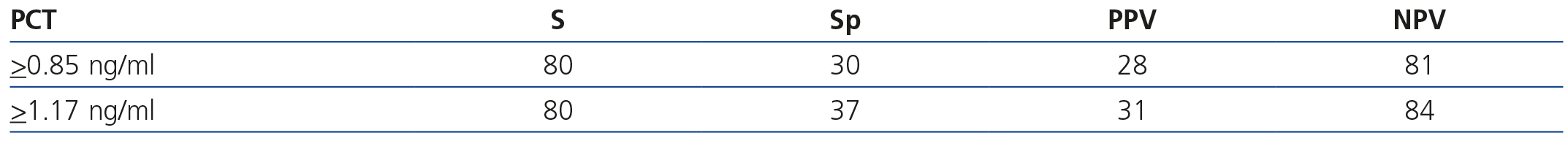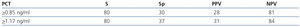Objetivo: Establecer la utilidad de la procalcitonina (PCT) y otros parámetros clínicos y analíticos como indicadores de daño renal agudo y permanente en niños tras una primera infección del tracto urinario (ITU) febril. Material y métodos: Estudio retrospectivo multicéntrico. Estudio estadístico: descriptivo, curvas ROC y regresión logística múltiple. Resultados: 219 pacientes, con edades entre 1 semana y 14 años (68 % menores de 1 año). Las medias de PCT fueron significativamente mayores en pacientes con pielonefritis aguda respecto a aquellos con DMSA agudo normal (4,8 frente a 1,44; p = 0,0001), sin alcanzar significación para DMSA tardío (6,5 frente a 5,05; p = 0,6). El área bajo la curva ROC de PCT fue 0,64 (IC 95 % 0,55-0,72) para daño renal agudo y 0,62 (IC 95 % 0,44-0,80) para permanente; con puntos de corte óptimos de 0,85 y 1,17 ng/ml. El análisis multivariante para daño renal agudo solo encontró correlación con PCT (odds ratio [OR] 1,2, IC 95 % 1,06-1,4; p = 0,005) y horas de fiebre (OR para < 6 h 0,4, IC 95 % 0,2-1,02; p = 0,05). En los pacientes con cicatriz, la OR para PCT fue 1,0 (IC 95 % 0,9-1,1; p = 0,6). Conclusiones: La PCT y la duración de la fiebre fueron los únicos parámetros que se asociaron de forma significativa a daño parenquimatoso agudo. No se observó relación estadísticamente significativa entre la PCT y la cicatriz renal.
Objective: To establish the utility of procalcitonin (PCT) and other clinical and analytical parameters as markers of acute and permanent renal damage in children after a first febrile urinary tract infection (UTI). Methods: Retrospective multicentre study. Statistical study: descriptive, receiver operating characteristic (ROC) curves and multiple logistic regression. Results: 219 patients, aged between 1 week and 14 years (68% under 1 year). The mean PCT values were significantly higher in patients with acute pyelonephritis with respect to normal acute DMSA (4.8 vs 1.44; p=0.0001), without achieving that signification for late affected DMSA (6.5 vs 5.05; p=0.6). The area under the ROC curve for PCT was 0.64 (CI 95% 0.55-0.72) for acute renal damage, and 0.62 (CI 95% 0.44-0.80) for permanent damage, with optimum statistical cut-off values of 0.85 and 1.17ng/ml. Multivariate analysis for acute renal damage only found correlation with PCT (Odds Ratio [OR] 1.2 (CI 95% 1.06-1.4, p=0.005), and hours of fever (OR for less than 6 hours of fever 0.4 (CI 95% 0.2-1.02, p=0.05). In patients with renal scarring, PCT showed an OR 1.0 (CI 95% 0.9-1.1, p=0.6). Conclusions: PCT and the duration of fever were the only parameters statistically associated with early renal damage. PCT and renal scarring did not reach statistical significance.
INTRODUCTION
Urinary tract infection (UTI) is one of the most common bacterial infections in children, with an overall incidence of 3%-7%1-5.
Lack of symptom specificity in younger patients, frequent association with malformations of the urinary tract and the possibility of permanent renal damage require multiple complementary tests in daily practice.
There is no consensus in clinical practice protocols and guidelines concerning the complementary examinations that should be carried out following a first episode of febrile UTI and which patients are at a greater risk of developing renal scarring4-8.
Currently, renal scintigraphy with Tc 99m dimercaptosuccinic acid (DMSA) is the gold standard for diagnosing acute and permanent kidney damage9,10. However, this test is expensive, is not available in all centres and requires the patient to undergo radiation. Consequently, there is an attempt to find an indicator that limits invasive tests to those patients at greater risk.
Various molecules have been proposed as possible markers for renal damage. Several studies have shown their usefulness for interleukin (IL)-6, IL-8 and urine osmolality, a more discreet usefulness for α tumour necrosis factor, N-acetylglucosamine and IL-16, and low or no usefulness for other tubular proteins11-13.
In recent years, the most studied marker was procalcitonin (PCT), due to its rapid and specific response to serious bacterial infections14,15. It is a precursor of calcitonin without hormone activity, with practically undetectable values in physiological conditions and during viral infections, and which increases rapidly and proportionally in response to bacterial infection and its severity. The increase occurs 2 hours after the onset of the infection, reaches its maximum level at 12 hours and normalises in 2-3 days when the infection has subsided.
There are diverse studies concerning the usefulness of PCT for diagnosing acute and chronic renal damage, whose results are contradictory16-27. The objective of our study was to assess the usefulness of PCT and other analytical (leukocytes, C-reactive protein [CRP], etc.) and clinical (age, hours of fever, etc.) parameters as indicators of acute and permanent renal damage in children after their first episode of febrile UTI.
MATERIAL AND METHOD
It is a retrospective multicentre study carried out in four hospitals in Valencia (Spain), collecting data from paediatric patients admitted during their first episode of febrile UTI, from 2006-2010.
Inclusion criteria were UTI diagnosis through positive urine culture with a significant number of colonies according to the collecting method, fever over 38ºC, leukocyte count above the upper value of the normal range according to age and/or CRP levels > 30 mg/l, with at least one PCT measurement and previous early or late DMSA.
Those patients with known uropathy (including prenatal ectasia) and those with a previous febrile UTI episode were excluded.
The study was approved by the Ethics Committees of the participating hospitals.
We recorded each patient’s clinical data (age, sex, fever duration until blood analysis, highest fever reached and related symptoms), the antibiotic treatment used and the hours it took the patient to become afebrile following the onset of that treatment. Analytical data included: leukocyte and neutrophil count, CRP and PCT values, urine collection method, bacterium identified in the urine culture, antibiotic sensitivity and the same bacterium’s presence or absence in the haemoculture.
The method for measuring PCT during the first years of the study was semi-quantitative using the immunochromatographic BRAHMS test (36 patients), and later using the BRAHMS Kryptor compact electrochemiluminescence immunoassay method (183 patients). Only quantitative PCT values were used for the statistical analysis.
Regarding the imaging tests, we collected results from the conventional ultrasound and acute and/or late phase DMSA.
The scintigraphic study was performed 3 hours after injecting a weight-adjusted TC-DMSA dose, obtaining three planes. The presence of focal or diffuse areas of uptake defects, without evidence of cortical loss, was considered pathological.
In those cases in which cystography was performed (either early pathological DMSA or dilation of the upper urinary tract detected in ultrasound during admission), we recorded the presence or absence of vesicoureteral reflux (VUR), the degree and the uni/bilaterality.
Statistical analysis included the calculation of sensitivity (S), specificity (Sp), positive predictive value (PPV), negative predictive value (NPV) and calculation of the area below the ROC curve. The χ2 test and the Student’s t-test were performed to compare the categoric and quantitative variables, respectively. Non-conditional logistic regression was used to estimate the association between the variables of interest.
RESULTS
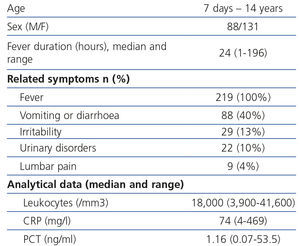
A total of 219 patients (131 girls and 88 boys) were included in the study, aged between 1 week and 14 years, of which 149 (68%) were younger than 1 and 18 were younger than 28 days (8.2%).
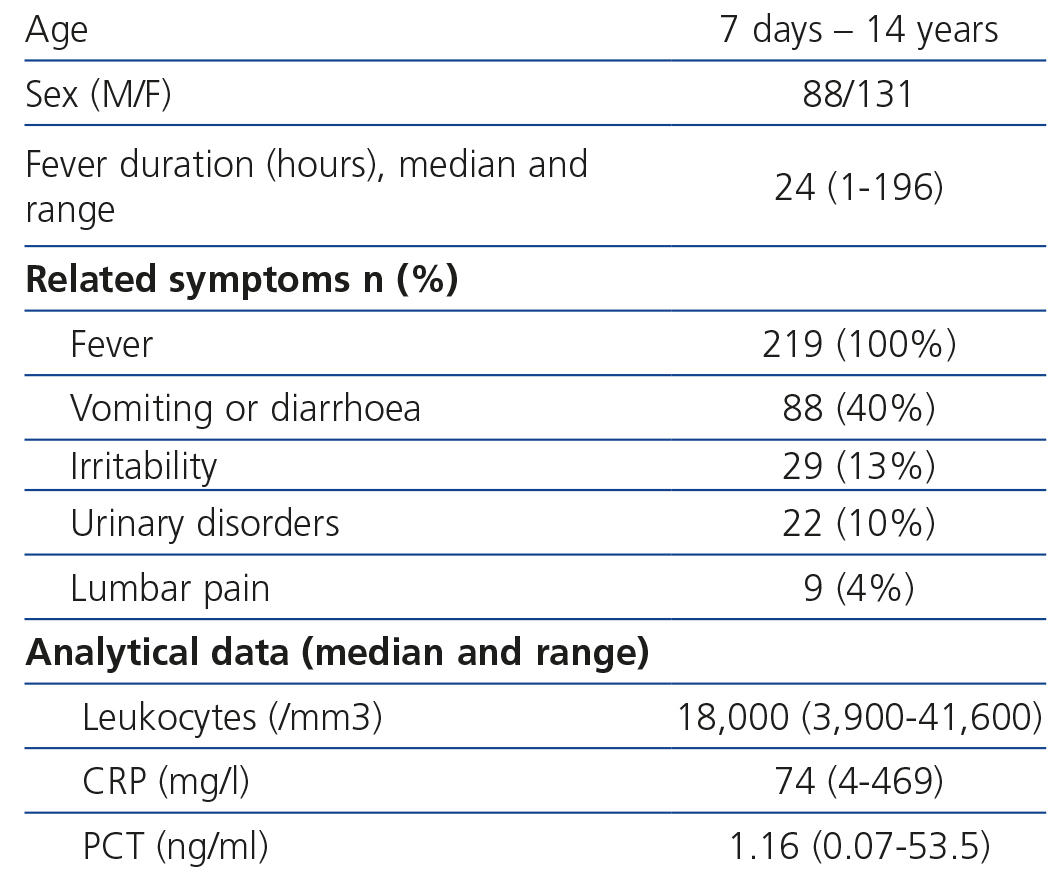
The median value of the fever’s development in hours when performing blood analysis was 24 [1-96] and the most common associated symptoms were gastrointestinal. As regards the analytical results, the leukocyte median was 18,000/mm3 (3,900-41,600), CRP median 74mg/l (4.469) and PCT median 1.16ng/ml (0.07-53.5) (Table 1).
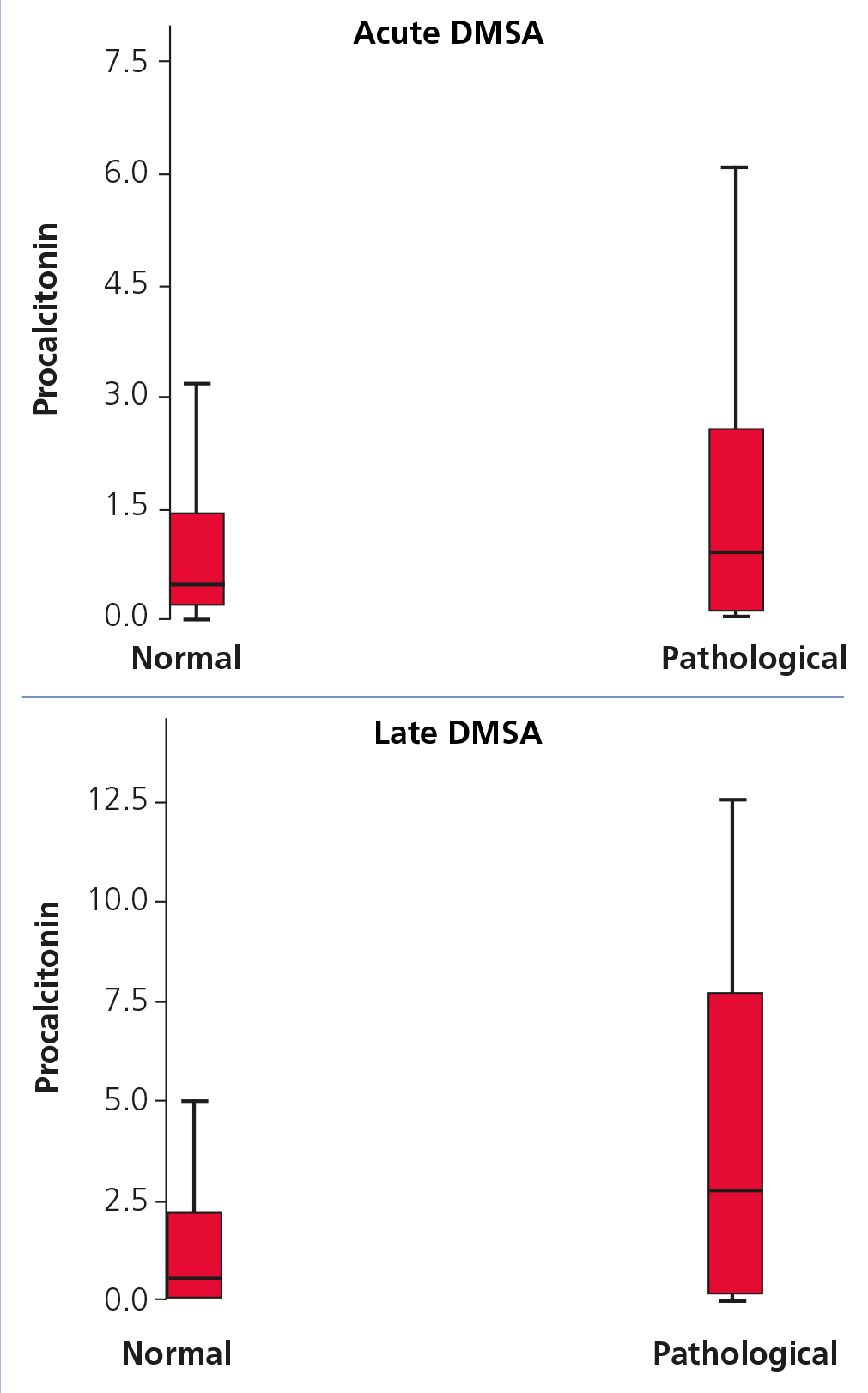
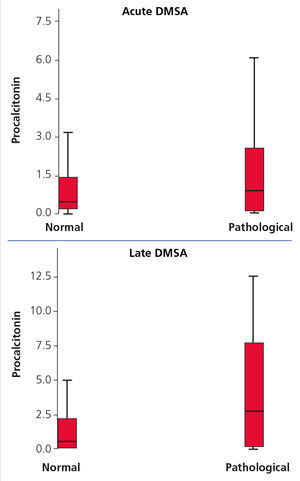
The tendency for PCT medians to increase in patients with normal and pathological DMSA, both in acute and late phase, is shown in Figure 1.
The urine collection method in continent patients was via mid-stream sampling. An adhesive bag was used on 60 incontinent patients (35%), always obtaining two samples, and a sterile technique was used on the remainder (urinary catheterisation or suprapubic aspiration).
The most common isolated bacterium was Escherichia coli in 92.7% of cases.
Other bacteria were isolated in 16 patients (7.3%): 4 klebsiella (25%), 3 Proteus, 3 enterobacteriaceae, 2 enterococci and 2 Citrobacter and 2 others.
The antibiotic guideline chosen in the majority of cases was intravenous gentamicin (65%), with a mean fever disappearance time of 32 hours after the onset of treatment.
Conventional ultrasound was performed on all patients, with 60 (27%) presenting disorders. Doppler Sonography was carried out simultaneously in some centres (72 sonographs, 22 pathological alterations).
Acute-phase DMSA was performed on all patients in the first 7 days after admission (late DMSA alone was performed on 2 patients); 142 patients presented alterations (64.8%). In the cases with infection by a bacterium other than E. coli, the percentage of scintigraphic acute-phase alterations was 53%.
In those patients with early pathological DMSA, late DMSA was indicated a mininum of 9 months after performing the previous DMSA. The presence of renal scarring was interpreted in this context as secondary to acute damage, however these changes may not necessarily be due to the cause-effect of early injury. This was carried out on 99 patients, with 22 resulting pathological (22.8%).
Cystography was performed on 126 patients, of which 30 (23.8%) were diagnosed with VUR.
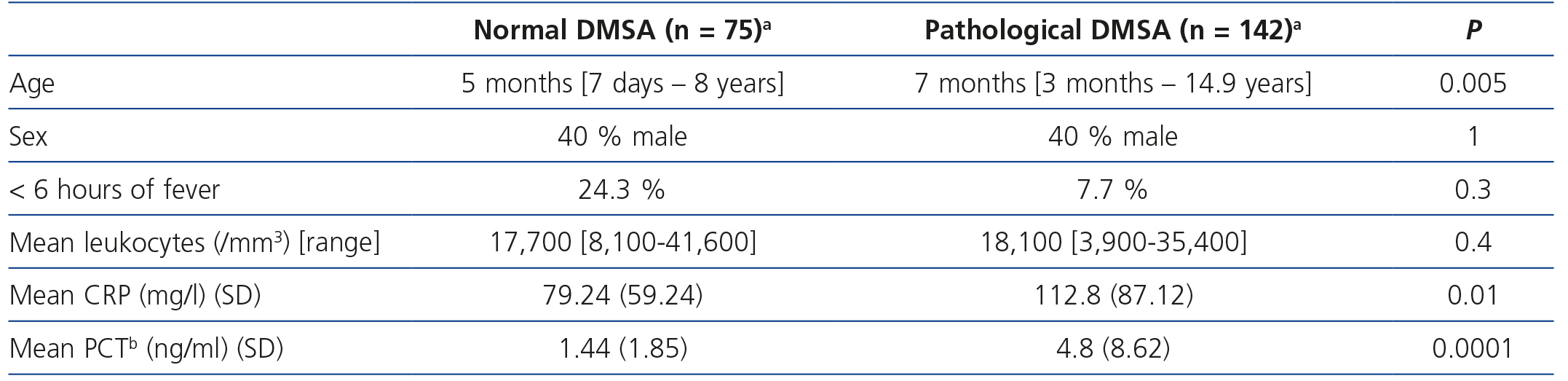
The most significant clinical and analytical data in children with acute-phase pathological DMSA (acute pyelonephritis) and in those with normal DMSA are recorded in Table 2. PCT means were significantly higher in patients with acute-phase pathological DMSA in respect to those unaffected (4.8 vs 1.44; P=.0001); this significance was not achieved for late DMSA (6.5 vs 5.05; P=.6). Similarly, CRP averages behaved with respect to early DMSA (112.8 vs. 70.24; P=.01) and late DMSA (163.39 vs. 115; P=.06).
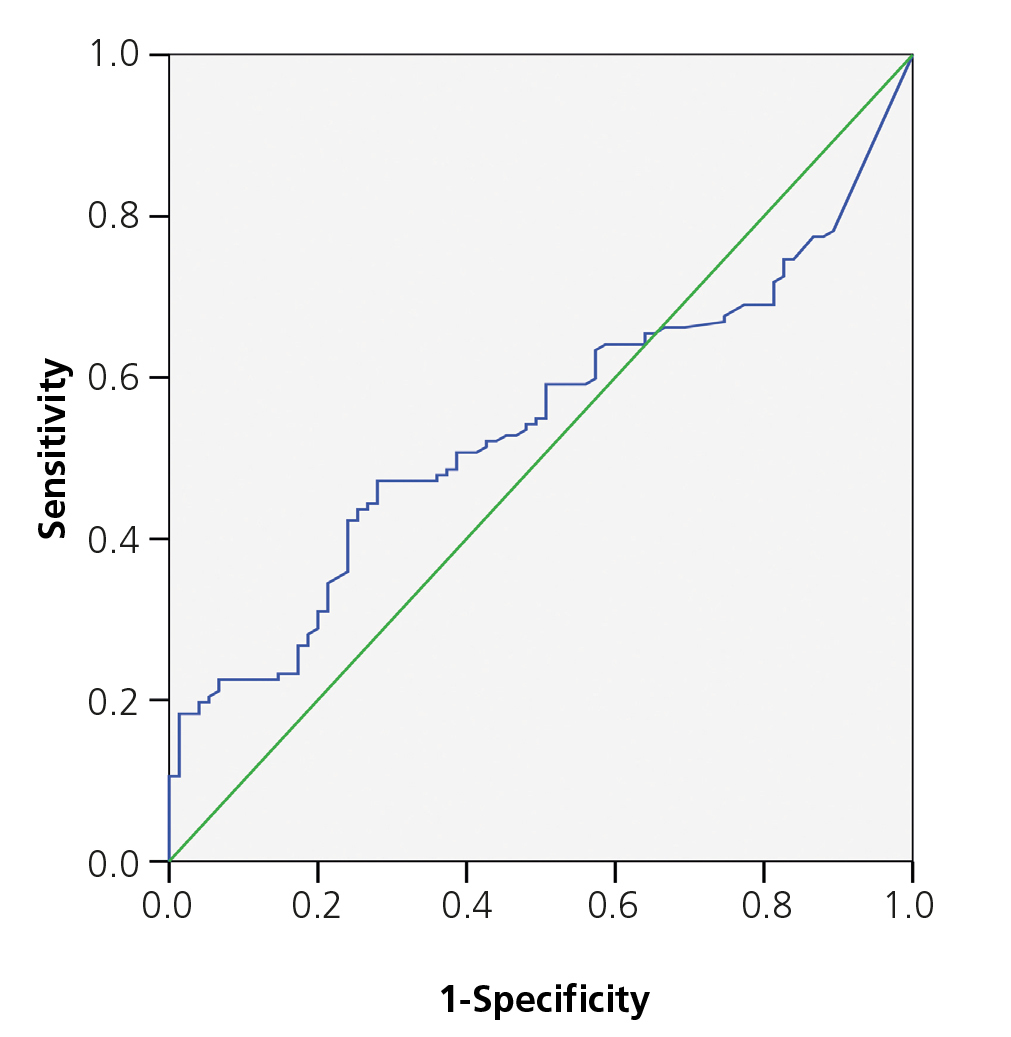
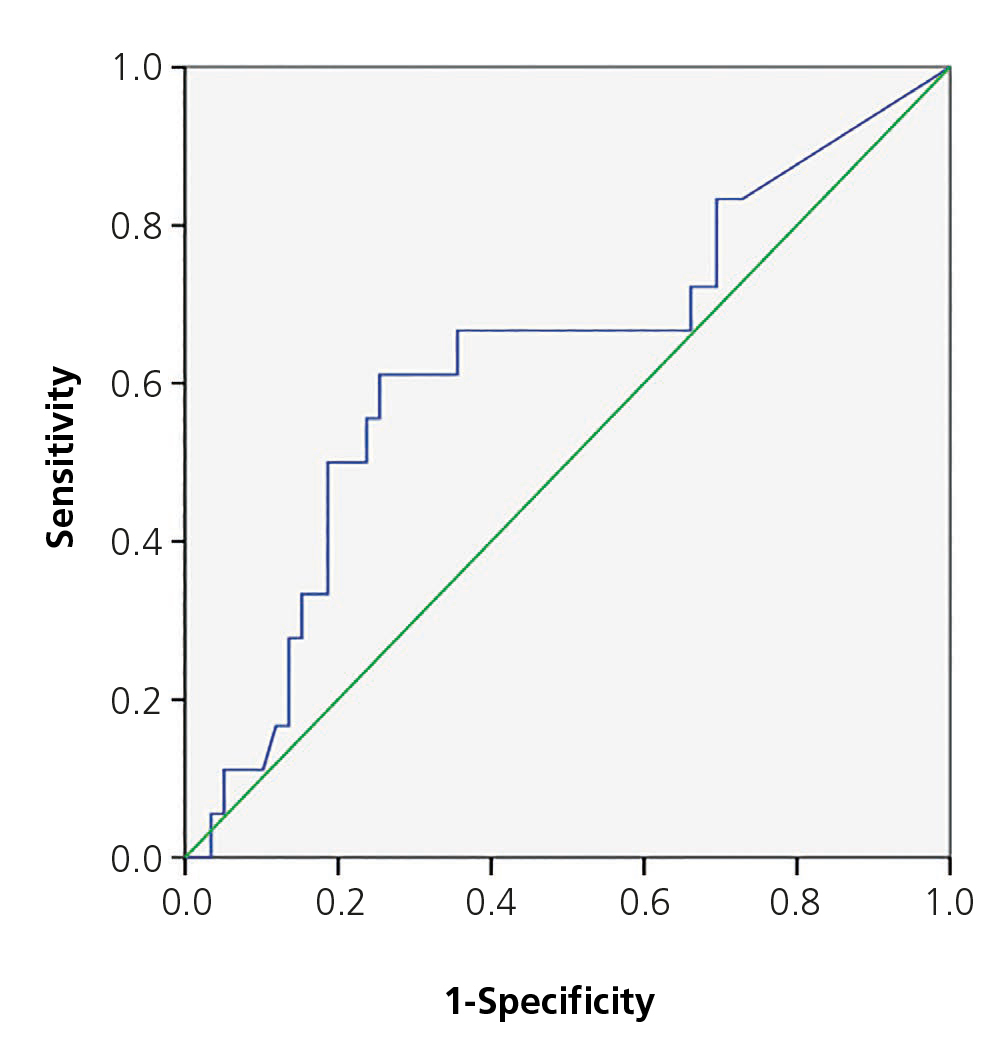
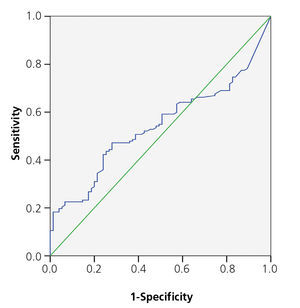
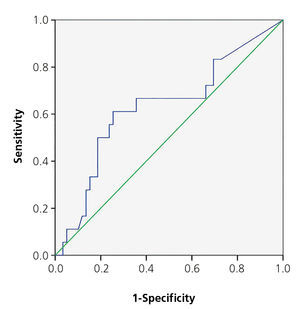
The area below the PCT ROC curve for acute renal damage was 0.64 (95% CI 0.55-0.72) and 0.62 for scarring (95% CI 0.44-0.80), establishing values of 0.85 and 1.17ng/ml as cut-off points (Figures 2 and 3). The values of S, Sp, PPV and NPV for acute and chronic renal damage are presented in tables 3 and 4, respectively.
The multivariate analysis with respect to acute renal damage only found association with PCT (Odds Ratio [OR] 1.2, 95% CI 1.06-1.4; P=.005) and fever duration (OR for <6h 0.4, 95% CI 0.2-1.02; P=.05). Patients younger than one year presented lower risk of scinitgraphic affectation, although statistic significance was not achieved (OR 0.52, 95% CI 0.2-1.1; P=.08).
No association with any of the studied factors was found in the assessment of permanent renal damage. Specifically, OR was 1.0 for PCT (95% CI 0.9-1.1, P=.6), and 0.4 in patients younger than one (95% CI 0.1-1.4; P=.1).
DISCUSSION
The aim of this study was to assess the usefulness of PCT and other analytical (leukocytes, CRP, etc.) and clinical (age, hours of fever, etc.) parameters as indicators of acute and permanent renal damage in children after their first episode of febrile UTI. Our study includes a considerably sized group with respect to those studies published to date of patients diagnosed with first episode of febrile UTI with high theoretical possibility of acute scinitgraphic affectation due to analytical alterations presented on admission (leukocyte count above the upper value of the normal range according to age and/or CRP levels >30mg/l). In addition, DMSA, the gold standard for renal damage diagnosis, both acute and permanent, according to general recommendations at the time of the test, was performed on all patients.
Although the characteristics of the newborn subgroup may differ to those of the rest of the study’s population, we decided to include this group to maintain the homogeneity of the participating centres and to guarantee, as far as possible, sufficient statistical power in order to establish statistically significant associations.
Despite the causal bacterium being E. coli in the majority of patients, other bacteria were discovered in 16 of the study population. It involves 8 males and 7 females, which constitute 7% of the sample’s total, of which 9 (56%) were younger than 1 year. Fever duration was <24 h (94%) in most of patients, and < 6 hours (37.5%) in 6 patients. Regarding the complementary tests, ultrasound was normal in 12 (75%) and PCT was negative in 10 of these patients (62%).
The percentage of acute-phase pathological DMSA was 64.8% in our series, very similar to the rest of the published studies16,17,19,24,26,28. Contrary to what was expected6, in the cases of infection by bacterium other than E. coli, the percentage of acute-phase pathological DMSA was lower (53%), with 3 developing scarring (19%). The clinical and analytical characteristics of this group of patients were analysed, without finding any risk or confusion factor justifying this result.
PCT has demonstrated its validity in the early diagnosis of invasive bacterial infections, surpassing CRP14,15. As regards UTI, PCT was the most studied marker and currently offers the best results. Since Benador published his study in 1998, numerous studies have tried to find a cut-off point for PCT with could be related to both risk of acute parechymatous damage and permanent scarring16.
The majority of publications, except for two, have shown the usefulness of PCT, although with very inconsistent S and Sp values17,18,19-27. Although sample sizes are often small, we highlight Leroy’s 2013 meta-analysis which assesses 18 independent studies, thus obtaining a total of 1,011 patients26. In this instance, PCT shows good results for acute renal damage and low Sp for renal scarring.
Our study, as well as providing a considerably larger sample size than the majority of previous publications, includes a multivariate analysis to independently determine the association between the variables of interest and renal involvement.
As regards acute renal damage, we found significantly higher PCT averages in patients with pathological DMSA. After calculating the area below the curve, optimal PCT values of 0.85 and 1.17ng/ml were found as cut-off points, which are similar to those proposed in other studies (1ng/ml in the studies by Sheu and by Bressan, and 0.85ng/ml proposed by Kotoula)24,23. However, our S, Sp, PPV and NPV values are much lower than those discovered in these studies.
With respect to renal scarring, the increase of PCT values did not achieve statistical significance. The area below the ROC curve was similar to that for acute damage, and for the same PCT values as cut-off points, we found acceptable values of S, but very low E and PPV values. Our data, with respect to the usefulness of PCT for diagnosing permanent renal damage, did not allow room for recommendations made by other authors16-27.
In reference to the other markers studied, only CRP achieved certain usefulness in the diagnosis of acute damage, similar to the study by Kotoula23.
The results of the multivariate analysis demonstrate that only two factors were relevant: PCT and <6 hours of fever. Each unit that increases PCT represents a 20% greater risk of acute parenchymatous damage. In contrast, <6 hour fever duration gave 60% protection.
Contrary to that expected, an age of <1 proved to be a protecting factor for acute and chronic parenchymatous affectation; however, statistical significance was not achieved.
The results of our study demonstrate certain usefulness of PCT for predicting acute renal damage in patients undergoing their first episode of febrile UTI. Although we cannot establish PCT cut-off values in those patients with low PCT levels, ultrasound normality and good initial evolution, the performance of acute-phase DMSA could be prevented.
However, data obtained until now does not allow the use of PCT as a predictor of renal scarring in these patients.
Our study has certain limitations. Firstly, it is a retrospective study. Second, the urine collection method in a significant percentage of cases was via perineal bag and not using a sterile technique, as recommended in the latest clinical practice guidelines. However, having two urine cultures, with growth of the same bacterium and in significant number, as well as urinalysis with inflammatory signs, minimises the possibility of false positives.
Finally, of the 142 patients with acute-phase DMSA, only 99 late DMSA were performed during the study, since in some cases sufficient time had not elapsed for it to be carried out (5 patients) or the initial scintigraphic affectation was mild and clinical evolution satisfactory (16 patients). Some patients were also lost to follow-up (22 cases, 10% of the sample). Due to this small sample of patients with renal scarring, we are not able to obtain concluding statistical data.
CONCLUSIONS
PCT increase and <6 hour fever duration were the only parameters which were significantly associated with acute parenchymatous damage. No statistically significant association was observed between PCT and renal scarring.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Description of clinical and analytical data
Table 2. Comparison between normal and pathological acute-phase dimercaptosuccinic acid
Table 3. Diagnostic validity of procalcitonin with respect to Tc 99m dimercaptosuccinic acid in acute damage
Table 4. Diagnostic validiy of procalcitonin with respect to Tc 99m dimercaptosuccinic acid in renal scarring
Figure 1. Procalcitonin values in patients with normal and pathological dimercaptosuccinic acid in acute phase (acute pyelonephritis) and late phase (scarring)
Figure 2. ROC curve for procalcitonin in acute kidney damage.
Figure 3. ROC curve for procalcitonin in renal scarring.