The choice of renal replacement therapy (RRT) is an important decision that determines the quality of life and survival. Most patients change from one RRT modality to another to adapt RRT to clinical and psychosocial needs. This has been called «integrated model of RRT» that implies new questions about the best sequence of techniques.
Material and methodsThe study describes the impact of transitions between RRT modalities on survival using the Madrid Registry of Renal Patients (2008–2018). This study used the proportional hazards models and competitive risk models to perform an intention-to-treat (ITT), according to their 1st RRT modality and as-treated (AT) analysis, that consider also their 1st transition.
ResultsA total of 8971 patients started RRT during this period in Madrid (6.6 Million population): 7207 (80.3%) on hemodialysis (HD), 1401 (15.6%) on peritoneal dialysis (PD) and 363 (4.2%) received a pre-emptive kidney transplantation (KT). Incident HD-patients were older (HD group 65.3 years (SD 15.3) vs PD group 58.1 years (SD 14.8) vs KTX group 52 years (SD 17.2); p < 0.001) and had more comorbidities. They presented higher mortality (HD group 40.9% vs PD group 22.8% vs KTX group 8.3%, p < 0.001) and less access to a transplant (HD group 30.4% vs PD group 51.6%; p < 0.001). Transitions between dialysis techniques define different groups of patients with different clinical outcomes. Those who change from HD to PD do it earlier (HD → PD: 0.7 years (SD 1.1) vs PD → HD: 1.5 years (SD 1.4) p < 0.001), are younger (HD → PD: 53.5 years (SD 16.7) vs PD → HD: 61.6 years (SD 14.6); p < 0.001), presented less mortality (HD → PD: 24.5% vs PD → HD: 32.0%; p < 0.001) and higher access to a transplant (HD → PD: 49.4% vs PD → HD: 31.7%; p < 0.001). Survival analysis by competitive risks is essential for integrated RRT models, especially in groups such as PD patients, where 51.6% of the patients were considered as lost follow-up (received a KTX after during the first 2.5 years on PD). In this analysis, survival of patients who change from one technique to another, is more similar to the destination modality than the origin one.
ConclusionOur data suggest that transitions between RRT-techniques describes different patients, who associate different risks, and could be analyzed in an integrated manner to define improvement actions. This approach should be incorporated into the analysis and reports of renal registries.
La elección del tratamiento sustitutivo renal (TSR) es una decisión importante que determina la calidad de vida y la supervivencia. La mayoría de los pacientes cambiará de una modalidad de TSR a otra para adaptarla a sus necesidades dentro de lo que se conoce como modelo de TSR integrado. En estas circunstancias surgen nuevas preguntas sobre la mejor secuencia de técnicas o las consecuencias de las transiciones.
Material y métodosDescribimos las transiciones entre técnicas de TSR y su impacto en la supervivencia a partir del Registro Madrileño de Enfermos Renales (REMER), durante un periodo de 11 años. Se utilizaron los modelos de riesgos proporcionales y de riesgos competitivos para realizar un análisis por intención de tratar (ITT) según su 1.er tratamiento y como tratado (AT) considerando la 1.ª transición.
ResultadosUn total de 8.971 pacientes iniciaron su primer TSR durante este periodo en Madrid (6,6 millones habitantes): 7.207 (80,3%) en hemodiálisis (HD), 1.401 (15,6%) en diálisis peritoneal (DP) y 363 (4,1%) recibieron un trasplante renal anticipado (TXR). En el análisis ITT, los pacientes incidentes en HD eran mayores (HD 65,3 años (DE 15,3) vs. DP 58,1 años [DE 14,8] vs. TXR 52,0 años (DE 17,2); p < 0,001) y tenían más comorbilidades. Presentaron mayor mortalidad (HD 40,9% vs. DP 22,8% vs. TXR 8,3%, p < 0,001) y menor acceso a trasplante (HD 30,4% vs. DP 51,6%; p < 0,001). Las transiciones entre las técnicas de diálisis identifican diferentes fenotipos de pacientes con diferentes resultados clínicos en el análisis AT. Los pacientes que cambiaban de HD a DP lo hacían más precozmente (HD → DP: 0,7 años (DE 1,1) vs. DP → HD: 1,5 años [(DE 1,4); p < 0,001), eran más jóvenes (HD → DP: 53,5 años (DE 16,7) vs. DP → HD: 61,6 años, (DE 14,6) p < 0,001), sufrían menor mortalidad (HD → DP: 24,5% vs. DP → HD: 32%, p < 0,001) y tenían mayor acceso al TXR (HD → DP: 49,4% vs. DP → HD: 31,7%, p < 0,001). El hecho de que accedieran más al TXR modifica la probabilidad de alcanzar el evento analizado (mortalidad) y actúa como un riesgo competitivo. En este análisis, la supervivencia de los pacientes que cambian de una técnica a otra se parece más a la de la modalidad de destino que a la de origen.
ConclusiónNuestros datos sugieren que las transiciones entre técnicas describen diferentes perfiles de pacientes, con distintos riesgos asociados y deben analizarse de manera integrada para definir acciones de mejora. Este enfoque podría incorporarse en el análisis y los informes de los registros renales.
Every year more than 120,000 Americans,1 83,000 Europeans2 and 6500 Spaniards3 progress to the last phase of their chronic kidney disease (CKD) and start dialysis or transplantation ( KT). The choice of renal replacement therapy (RRT) is an important decision that affects quality of life, intercurrent events, and patient survival4. CKD patients on dialysis have a 10-fold higher risk of death than the general population,5 with notably higher hospitalization rates and poorer health-related quality of life6,7. It is striking that the risk of mortality is not evenly distributed over time and appears to be higher during the first 3 months on RRT8.
The search for the ideal RRT technique has guided the conventional approach of comparative analysis of results as if modalities of therapy were independent fields; however, clinical reality shows that many patients will use different modalities depending on their circumstances at any given time.9,10 This paradigm is defined as the “integrated RRT model” because it is intended to consider treatment routes rather than individual RRT techniques. Some studies show that there are differences depending on the sequence chosen for the RRT modality changes.11,12
In these circumstances, it is inevitable to abandon the dichotomous hemodialysis (HD) vs peritoneal dialysis (PD) approach and ask ourselves new questions about the optimal sequence of techniques or the ideal length of stay in the different modalities to plan the transitions between the different RRT therapies.13–15 The available literature suggests that, at present, the transition between the different modalities is poorly coordinated, which could explain the significant increase in morbidity and mortality.16,17
In Spain, CKD care is universal and depends on the public health system, which guarantees a free choice of RRT, only limited by the technical contraindications of each case. In addition, our system collects the basic information of the RRT in official registers, of obligatory completion, which guarantees the exhaustiveness, precision and validity of the data. However, the registry reports usually analyze the techniques independently, attributing mortality to the technique present at the time of the event, without considering the transitions in the complete follow-up of the patient.
The main objective of this work is to describe the movement of patients between the different RRT techniques. We will also analyze survival and sociodemographic data associated with RRT sequences, which imply staying or making transition between therapies.
MethodsStudy populationObservational, descriptive study, in which we analyzed all incident patients older than 16 years of the Madrid Registry of Renal Patients (REMER) starting RRT during the period between January 1, 2008 and December 31, 2018 without excluding any patient starting RRT during this study period. According to the definitions of the GRER (Group of Registries of renal patients), an incident patient is considered to be anyone who starts any of the dialysis or transplant techniques for the first time, and any subsequent change in technique will be considered a transition. Thus, for example, the KTof a patient on HD would not be an incidence in KTbut transfer or change of technique, the same would occur in a patient who started dialysis after KTdysfunction. The REMER cross-references its information with the database of the National Transplant Organization (ONT) and the National Institute of Statistics (INE in spanish), to refine mortality and follow-up inconsistencies.
Hospitals in the Community of Madrid are classified into three groups according to their portfolio of services and degree of complexity of clinical attention according to the criteria of the General Directorate of Specialized Care.18 At the level 3 or high complexity are the centers with a complete service portfolio that perform some type of transplant. Hospitals with a broad portfolio of services, but without transplants, belong to level 2 or intermediate complexity, and centers with a reduced portfolio of services belong to level 1 or low complexity.
Exposure variable / OutcomeThe main variable analyzed is the state in which the patient has been in throughout the follow-up time. These states represent the different types of RRT: HD, PD, and the KT.
The transitions are defined as the progressions between the different available treatments until the final event of death of the patient, and reflect the evolution of advanced kidney disease.
All the variables included in the description of the REMER are included: demographic variables, kidney disease, type of RRT and outcome.19 It is performed a descriptive analysis of the integrated evolution of the different groups of patients either always staying in one technique or changing between them. We analyzed the initial RRT modality relative to the degree of complexity of the hospital and dialysis centers.
As a secondary objective of interest, we described the causes of mortality for each of the groups.
Statistical analysis is initially performed by intention to treat (ITT) and it categorizes the patients according to the baseline technique; in this way, transfers between techniques are prevented from influencing the results. Next, an analysis is performed by the RRT received up to the time of the event, as-treated (AT), to study whether the results vary depending on the changes in modality of RRT. In this way, we defined four subgroups based on the different dialysis transition situations until KT or the death event: only HD, only PD, start HD and go to PD or start PD and go to HD.
Statistical analysisThe results are expressed as percentages, mean and standard deviation (SD) or median and interquartile range (IQR) according to whether data is following a normal distribution and the nature of the variables. Comparisons between categorical variables are made with chi-square and between quantitative variables with Student’s t or Mann–Whitney U depending on the distribution of the sample. For the analysis of time-dependent events (survival, maintenance in technique), the results have been estimated using the Kaplan–Meier (KM) method and the log-rank test to assess statistical significance. Survival estimates are made by ITT, considering the first RRT performed. To analyze the impact of transitions on survival, only the first transition between the different RRTs is considered.
Cox regression was used to define the multivariate risk models, including those significant covariates in the univariate model or those that modified the model with a pIN/pOUT 0.05/0.1.
Competing risk analysisWhen an event modifies the probability of obtaining the event of interest, proportional hazards models are not suitable for estimating the probability of survival (Kaplan Meier (KM) model, Cox). In this case, a competing risks model should be used, which, unlike the KM model, does not overestimate the real rate of the event20.
Regression analysis to assess competing risks has been used to determine the effects of different dialysis modalities and their transitions on mortality (main event) and KT (competing event). We have represented the curves of cumulative events (CIF, cumulative incidence failure), for the events of interest (mortality and KT). All analyzes were performed with Stata 14 (StataCorp2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP.X).
This project has been approved by the CEIm of the Hospital Universitario Puerta de Hierro-Majadahonda (NREF 132/16), by the REMER Technical Committee and authorized by the Ministry of Health of the Community of Madrid under the supervision of the Office of Security of Systems of Health Information (OSSI) as described in previous publications6.
ResultsDescription of the cohort according to initial renal replacement therapyA total of 8971 patients started RRT in the Community of Madrid during the 11 years of follow-up. The majority, 80.3% (7207), were started on HD while 15.6% (1401) started on PD and 4.1% (363) received pre-dialysis KT, with 33.3% of living donor. The percentage of patients who started home hemodialysis (HHD) was 0.3%.
Table 1 shows the characteristics of the patients classified by ITT, with a mean follow-up of 4.38 years (SD 3.08). Patients who started HD were older and had diabetic comorbidity and had a higher mortality. Patients on PD and those receiving KT in pre-dialysis have a higher proportion of patients with polycystic Kidney Disease or glomerular pathology; furthermore, those who received predialysis KTwere the youngest and had the lowest mortality.
Characteristics of incident patients receiving renal replacement therapy according to their initial technique.
| Overall (n = 8971) | HD (n = 7207) | PD (n = 1401) | KT(n = 363) | p Value | |
|---|---|---|---|---|---|
| Age (years); mean, SD | 63.6 (15.7) | 65.3 (15.3) | 58.1 (14.8) | 52.0 (17.2) | <0.001 |
| Male (%) | 66.4 | 67 | 64.4 | 62.8 | 0.06 |
| Etiologies (%) | <0.001 | ||||
| Vascular/DM | 16.8/27.0 | 17.7/29.1 | 14.2/20.9 | 8.6/9.1 | |
| GN/INT | 14.3/8.7 | 12.4/8.3 | 21.5/9.4 | 23.8/15.5 | |
| PKD/hereditary | 7.6/1.7 | 6.2/1.2 | 12.3/3.6 | 17.4/5.3 | |
| Unknown/other | 9.4/14.5 | 10/15.1 | 6.6/11.5 | 8.6/11.7 | |
| Initial technique (%) | 0.5 HDD | 18.5 APD | 33.3 DLive | ||
| Hospital classification (%) | |||||
| Level 3 | 75.1 | 24.9 | <0.001 | ||
| Level 2 | 83.0 | 17.0 | |||
| Level 1 | 88.2 | 11.8 | |||
| Total follow-up (years) mean (SD) | 4.28 (3.08) | 4.09 (3.04) | 4.98 (3.14) | 5.52 (3.04) | <0.001 |
| Last RRT (%) | |||||
| HD | 60.7 | 71.6 | 18.8 | 5.0 | |
| PD | 6.5 | 1.4 | 34.0 | 1.7 | <0.001 |
| KT | 32.9 | 27.1 | 47.2 | 93.4 | |
| Final event (%) | |||||
| Death | 36.8 | 40.9 | 22.8 | 8.3 | <0.001 |
| Patients with some KT | 36.6 | 30.4 | 51.6 | 100 | <0.001 |
| Cause of death (%) | <0.006 | ||||
| CV | 37.1 | 36.2 | 43.5 | 34.6 | |
| Infection | 26.6 | 26.8 | 27.4 | 30.8 | |
| Tumor | 13.6 | 13.7 | 13.0 | 15.4 | |
| Others | 22.7 | 23.3 | 16.1 | 19.2 | |
Hospital classification: classification of the hospital according to the degree of complexity of the Community of Madrid.18 Level 3: high complexity hospitals; Level 2: intermediate complexity hospitals; Level 3: low complexity hospitals.
CV: cardiovascular; SD: standard deviation; DM: diabetes mellitus; PD: peritoneal dialysis; APD: automated peritoneal dialysis; DLive: living donor; GN: glomerulonephritis; HD: hemodialysis; HDD: home hemodialysis; INT: interstitial; PKD: polycystic kidney disease; RRT: renal replacement therapy; KT: kidney transplant.
A 40% of patients start RRT in high-complexity hospitals, which are reference centers for performing KT and have broader PD programs.
Of the 8608 incident dialysis patients, 30.4% (2190) of HD patients and 51.6% (723) of PD patients received KT during a follow-up of 4.09 years (SD 3.04) on HD and 4.98 years (SD 3.14) on PD.
If we analyze the technique in which the patients were at the end of their follow-up, most of those who were transplanted in pre-dialysis (93.4%) and those who started HD (71.6%) continued with the same technique. However, almost half of the patients who chose PD were transplanted at the end of follow-up, with only approximately one third remaining on PD (Table 1).
More than a third of patients died during an overall follow-up time in RRT of 4.28 years (SD 3.08), with the most frequent cause of death being cardiovascular (37.1%). In our series, patients who received KT initially died almost 5 times less than those who started on HD and 2.4 times less than those who started on PD (mortality on HD of 40.9% during a follow-up of 4.09 years, SD 3.04; mortality in PD of 22.7% during a follow-up of 4.98 years, SD 3.14 and mortality in KT of 8.3% in a follow-up of 5.52 years, SD 3.04).
Table 2 presents the characteristics of the patients according to the type of initial RRT and their first transition. It shows that the patients who only receive HD are the oldest in the group, with lowest rate of transplants and with the highest mortality; at the end of the analysis period, 41.6% of these patients had died. By contrast the patients who only underwent PD were the youngest, with the highest incidence of glomerular pathology and received most transplants, in addition to having the best survival in the series.
Description of the transitions of incident patients in renal replacement therapy.
| PD (n = 1051) | HD (n = 6954) | PD → HD (n = 350) | HD → PD (n = 253) | p Value | |
|---|---|---|---|---|---|
| Age (years); mean(SD) | 56.9 (14.7) | 65.7 (15.1) | 61.6 (14.6) | 53.5 (16.7) | <0.001 |
| Male (%) | 63.4 | 67.0 | 67.4 | 65.6 | 0.1 |
| Etiologies (%) | <0.001 | ||||
| Vascular/DM | 13.9/18.1 | 17.8/29.4 | 15.4/29.4 | 15.8/21.3 | |
| GN/INT | 23/9.1 | 12.1/8.4 | 16.9/10.3 | 20.2/6.3 | |
| PKD/hereditary | 13.1/4.4 | 6.1/1.2 | 10/1.14 | 7.5/1.2 | |
| Unknown/other | 6.2/12.2 | 10.1/14.9 | 7.7/9.2 | 7.1/20.6 | |
| Hospital classification (%) | |||||
| Level 3 | 18.7 | 71.3 | 6.2 | 3.8 | <0.001 |
| Level 2 | 12.7 | 80.0 | 4.2 | 3.0 | |
| Level 1 | 7.9 | 86.2 | 3.9 | 2.0 | |
| Dialysis centers/others | 3.2 | 94.3 | 0.5 | 1.9 | |
| Follow-up until first transition (years); mean (SD) | NA | NA | 1.5 (1.4) | 0.7 (1.1) | <0.001 |
| Change in 6 months (%) | NA | NA | 24.6 | 66 | <0.001 |
| Change in 12 months (%) | NA | NA | 44.9 | 84.2 | <0.001 |
| Last RRT (%) | <0.001 | ||||
| HD | 3.3 | 73.5 | 65.4 | 20.2 | |
| PD | 43.4 | 0.1 | 5.7 | 36.4 | |
| KT | 53.3 | 26.5 | 28.9 | 43.5 | |
| Final outcome: death (%) | 19.7 | 41.6 | 32.0 | 24.5 | <0.001 |
| Patients with someKT (%) | 58.2 | 29.7 | 31.7 | 49.4 | <0.001 |
| Cause of death (%) | <0.001 | ||||
| CV | 42.5 | 36.1 | 43.8 | 41.9 | |
| Infections | 26.1 | 26.3 | 20.5 | 25.8 | |
| Tumor | 14.0 | 13.5 | 13.4 | 11.3 | |
| Others | 17.4 | 24.1 | 22.3 | 21.0 | |
Hospital classification: hospital classification according to the degree of complexity of the Community of Madrid.18 Level 3: high complexity hospitals; Level 2: intermediate complexity hospitals; Level 1: low complexity hospitals.
CV: cardiovascular; SD: standard deviation; DM: diabetes mellitus; PD: peritoneal dialysis; PD → HD: patients who start peritoneal dialysis and switch to hemodialysis; GN: glomerulonephritis; HD: hemodialysis; HD → PD: patients who start hemodialysis and switch to peritoneal dialysis; INT: interstitial; NA: not applicable; PKD: Polycystic Kidney Disease; RRT: renal replacement therapy; KT: kidney transplant.
Regarding the possibility of changing modality, it is almost 7 times higher in patients who start PD (25.0%) than those who start HD (3.6%).
It is noteworthy that patients who start RRT in one modality and then switch to another resemble patients in the destination technique. Patients who switch from HD to PD, do it soon, in an average time of 0.7 (SD 1.1) years; furthermore, most transitions occur before the end of the first year. They are younger, have lower mortality and receive more KT than patients who are maintained in HD, and they tend to share more characteristics with patients who are only on PD.
Transference of patients from PD to HD occurs late after a mean of 1.5 (SD 1.4) years on PD. They have similar baseline characteristics to patients who only receive HD, although with a higher incidence of glomerular pathology.
Patients who only receive PD are the ones with the highest probability of survival. Thus, in those who start HD the mortality is twice HR 2.5 95% CI [2.2–2.9]; those who start PD and switch to HD have a mortality risk of 1.6 [1.3–2.0], and those who start HD and switch to PD the risk is 1.2 [0.9–1.6]. PD patients also receive more transplants, double that of those maintained in HD; 1.8 times more than those who go from PD to HD and 1.2 times more than those who start in HD and go to PD.
Description of transitions throughout the follow-upRegarding the number of transitions recorded per patient, 63% (5652) remained in the initial treatment throughout the analysis period and 29% (2612) changed treatment once. At the end of follow-up, 36.8% had died, 58.2% continued RRT, and 5% had a lost follow-up.
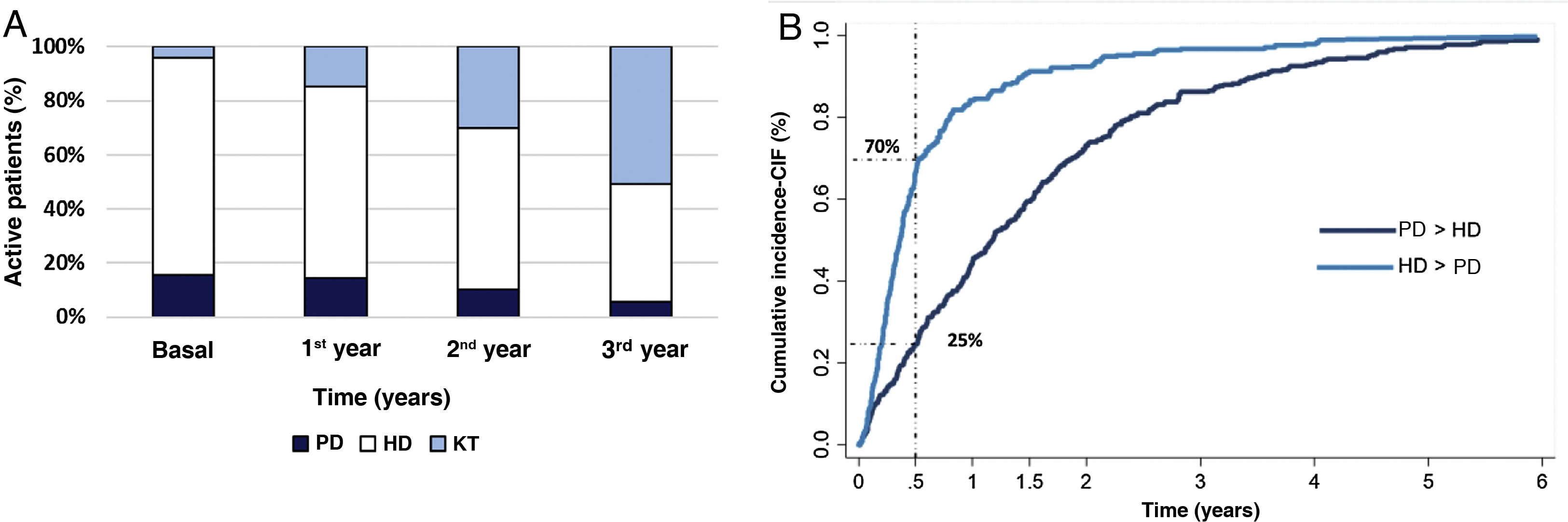
The most common technique used to initiate RRT is HD. As time goes by, KT becomes the most prevalent modality in active patients at the end of the third year of follow-up (Fig. 1A).
(A) Distribution of the RRT techniques of active patients at the end of each year. (B) Cumulative incidence curve until technique change for those patients who changed from PD to HD and from HD to PD.
PD: peritoneal dialysis; HD: hemodialysis; KT: kidney transplant. PD > HD: patients who start peritoneal dialysis and go on to hemodialysis. HD > PD: patients who start hemodialysis and switch to peritoneal dialysis.
Among the patients who change technique (Fig. 1B), those who start HD and switch to PD occurs early (66% of transfers occur before the first 6 months) while the switch from HD to PD is more gradual and lasts longer.
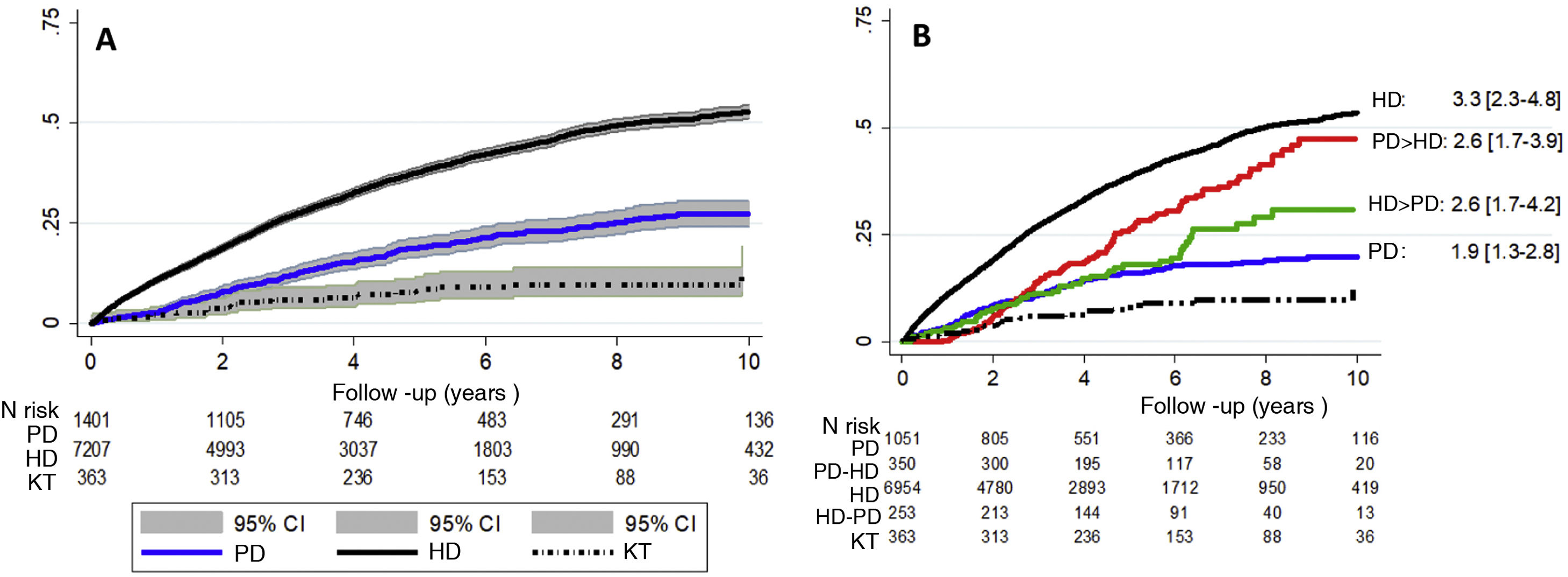
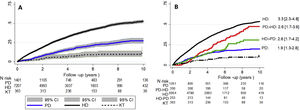
During a mean follow-up of 4.28 (SD 3.04) years, a total of 3303 deaths occurred. In the ITT analysis, after correcting for age, sex and diabetes, survival is higher in patients who initially receive KT , followed by patients on PD and lastly those on HD (Fig. 2A).
A comparison of all-cause mortality between the different forms of RRT and their transitions at the time of the as-treated event, with KT being the reference technique (Fig. 2B), it is observed that patients who receive only HD have higher mortality risk than the rest of the groups during the period of analysis.
Cumulative incidence curve for all-cause mortality; (A) according to the Kaplan–Meier model by intention to treat and according to baseline RRT; (B) according to treatment carried out in relation to the RRT sequence. The HR is adjusted for age, sex and diabetic nephropathy, with KT being the reference technique (HR = 1).
PD: peritoneal dialysis; HD: hemodialysis; KT: kidney transplant. PD > HD: patients who start peritoneal dialysis and switch to hemodialysis. HD > PD: patients who start hemodialysis and switch to peritoneal dialysis. CI: confidence interval; HR: hazard ratio.
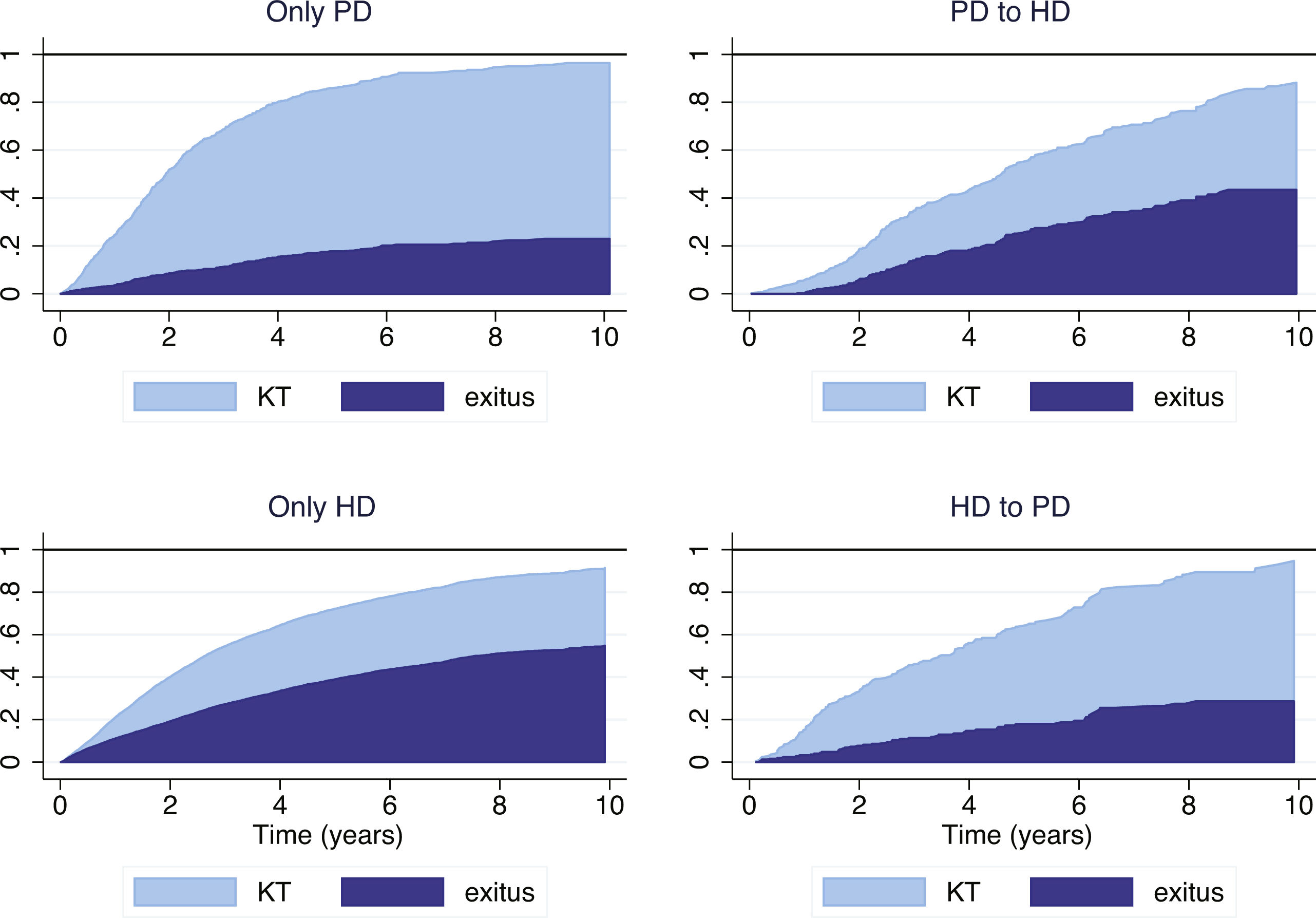
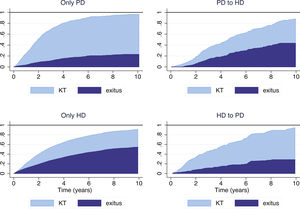
In our series, a high percentage of patients underwent transplantation, especially in the group with PD-only (58.2%) and those switched from HD to PD early (49.4%), which modifies the probability of reaching the mortality event which is the focus of the analysis. For this reason, it is most appropriate to analyze mortality considering KT as a competing risk and compare the subgroups of evolution in predefined RRT: only PD, only HD, PD to HD and HD to PD (Fig. 3). These graphical representations show that the mortality of patients who start RRT in one modality and then change tend to be similar to the patients of the destination technique rather than to those of the original technique.
Cumulative incidence curve (CIF) according to the starting technique and the first change in treatment (as-treated).
PD: peritoneal dialysis; HD: hemodialysis; KT: kidney transplant. PD > HD: patients who start peritoneal dialysis and switch to hemodialysis. HD > PD: patients who start hemodialysis and switch to peritoneal dialysis.
This is the first time that the consequences of changes in technique of RRT have been described in detail, showing the temporal sequence in each patient (percentage of change, time spent in each technique and characteristics of the patients in each group). This provides a new vision of RRT as an integrated process and illustrates the complexity of the analyzes that compare results from different dialysis techniques. This is an aspect that was insufficiently detailed in the previous reports of our regional registry and in the annual presentation of the REER.
The analysis are performed from two points of view: by ITT, which includes patients according to the RRT technique initially selected (HD, PD or KT ), regardless of whether they changed technique or not during the analysis period, and a as-treated (AT), which classifies patients according to the treatment they received and not according to the one initially assigned. In this case, the changes between HD and PD are considered and are divided into four groups: only PD, only HD, PD that changes to HD and HD that changes to PD.
In the ITT analysis, it is evident that the best survival in our series was that of the patients who received a TRX in pre-dialysis, consistent with evidence shown in other previous works21–23. However, only 4.1% of our patients received a TRX in a pre-dialysis situation; this is due to the unavailability of organs and comorbidities that forces the patient to start replacement therapy with PD or HD.
If mortality is analyzed without adjusting for the modality of initiation (Fig. 2A), there is evidence of greater survival in the group that starts with PD as compared to those that start on HD; this coincides with what has been previously described and it is probably due to the fact that, in the Spanish model, inclusion in PD presents a positive selection of the younger patient with less comorbidity24.
In the as-treated analysis, we evaluated the mortality associated with the transitions between modalities adjusted for age, sex, presence of diabetes mellitus, considering KT as the reference technique. In our study, multivariate analysis reveals that treatment exclusively with HD is associated with higher mortality and with less access to KT, as evidenced in the model that evaluates competing risks (Fig. 3). This could be due to the fact that the groups is made up of older patients who could have greater comorbidity. In contrast, the group of patients who have a longer long-term survival and who undergo the most transplants are those that remain on PD without going to HD; These results are consistent with what has been published previously,25 where PD patients have a high rate of KT due to their demographic characteristics and privileged clinical situation.
As for the transition from PD to HD, there seem to be two stages. An early stage in the first 3–6 months after the start of PD, in which a greater involvement of catheter-related dysfunction has been described,26,27 and a later stage due to failure of the technique. Our registry does not include a detailed report of the causes of exits, but there are studies such as those by Htay et al.28,29 who point out as risk factors for PD failure: a smaller center size, less use of icodextrin, insufficient control of serum phosphate, low use of automated peritoneal dialysis and use of antifungal prophylaxis. We know that the survival of the PD technique decreases significantly after 2 years, and since the waiting time for KT can be long, the change to HD is found to be frequent.30 In our study, the switch from PD to HD use to be late (a median of 14 months). These patients represent a profile of complex patients who come off PD late due to complications or exhaustion of the technique. It is noteworthy that the baseline characteristics of these patients who switch from PD to HD are similar to those who only receive HD in terms of age, diabetic nephropathy and possibilities of KT; however, survival is higher in patients who start on PD and switch to HD than those who only receive HD. This result could be related to the information provided from some series that describe an initial survival advantage of PD compared to HD,4,31,32 in addition to a greater knowledge of self-care, better maintenance of residual renal function, greater patient satisfaction and preservation of vascular access for the future.33
Patients who start HD and switch to PD do it early, most of them within the first 6 months, and the competitive survival is similar to those who started directly on PD. They are formed by the youngest patients in the study and have a lower incidence of diabetic nephropathy than patients exclusively on HD. We think that they could represent patients with an unscheduled start who are offered PD after starting HD. Late transfer from HD to PD seems to be due mainly to a failure of the technique, and it is associated to a higher risk of mortality17,34–36.
Limitations and strengthsIt is known that patients who start RRT without a previous schedule and often without having a follow-up in the low clearance outpatient clinic, usually initiate HD through a temporary catheter.37 Both, HD and temporary catheters have been associated with higher mortality38–40 and complications such as bacteremia, prolonged hospitalization,41 thrombosis, or central venous stenosis. The REMER registry, the basis of this work, does not include comorbidities or analytical parameters, so we cannot correct our results by these data. On the other hand, our objective is not to analyze the superiority of one form of dialysis over another, but to analyze RRT as an integrated treatment consisting of a sequence of different therapies, in which the initial technique, how and when the modality of treatment is changed could influence the evolution of the patient receiving RRT.
The characteristics of the population of the Community of Madrid may not be the same as in other communities, which limits the extrapolation of our results to other populations. However, one of the advantages of this work is that analyzes 100% of incident patients on RRT in our setting. The analyzed data comes from an official database, that is mandatory to complete and maintained in the historical series, in which internal validity is guaranteed.
Finally, our study is the first to take into account competitive risks when analyzing the evolution according to the initially modality of RRT together with the changes between the different techniques. This is especially relevant since, in the presence of these, KM survival estimates may not be correct and Cox models may lead to imprecise interpretations.
Improvement opportunitiesThe decision-making process that leads to the choice of a RRT is complex and may be influenced by social circumstances and the health education received by the patient.42 It is known that the lack of preparation of the patient with advanced chronic kidney disease (ACKD) and an urgent initiation of dialysis is associated with lower survival and higher mortality.43,44 With adequate support and pre-dialysis training, it has been estimated that up to 50% of ACKD patients are able to perform self-care dialysis with PD or HDD45. We do not have the reasons why the technique is changed, but we have been able to observe, in our sample, that 66% of the patients who go from HD to PD do so in the first 6 months of treatment. These patients with an early change in their treatment could represent a late choice of technique in patients with urgent initiation of RRT or lack of prior preparation. The timely referral of patients with advanced kidney disease to Nephrology services, and the continued development of ACKD units, could improve these results.
ConclusionsOur data suggest that the transitions between techniques are observed in patient with different profiles, with different associated risks, and that they should be analyzed in an integrated manner to define improvement actions. This approach could be incorporated into the analysis and reporting of renal registries. The data presented here may demonstrate that not only the initial choice of technique, but also how and when the modality is changed, could influence the evolution of the patient receiving RRT. Timely interventions and a change in modality at the right time could help mitigate risks in such a vulnerable group. More complex analyzes such as competitive risks and tracking the change in technique provide a more realistic view of RRT. We need additional studies that inquire into the factors associated with changes in techniques in order to offer strategies that improve overall results in kidney health.
FinancingThis study has been funded by the Instituto de Investigación Segovia-Arana-Puerta de Hierro-Majadahonda, Fundación Iñigo Álvarez de Toledo and Fundación Madrileña de Nefrología.
Conflict of interestsThe authors declare that they have no conflict of interest with this article. These results have not been previously reported.
We appreciate the collaboration of the REMER registry committee and especially his secretary, Mr. Manuel Aparicio, as well as all the clinical services and their staff who, with their daily work, contribute to the generation and maintenance of the REMER registry.