To analyze the results of endovascular treatment of venous anastomotic stenosis (VAS) in humero-axillary arteriovenous grafts (HAG), comparing outcomes between patent and thrombosed HAG.
Material and methodsA retrospective cohort study was made of endovascular treated patients because of a VAS in a HAG between January 2009 and December 2019. Group A: Thrombosed HAG secondary to a VAS. Group B: Patent HAG with a VAS detected during follow-up. Technical success was defined as residual stenosis after treatment <30%, and clinical success as satisfactory immediate dialysis after surgery. After ET a biannual clinical and ultrasound follow-up was performed. Statistical analysis: Survival analysis was performed for time-to-event data to assess patency.
ResultsGroup A: 55 patients. Group B: 22. There were no significative differences in demographic and anatomical factors between groups.
Technical and clinical success were 100% in group B and 94.5% and 91% respectively in group A.
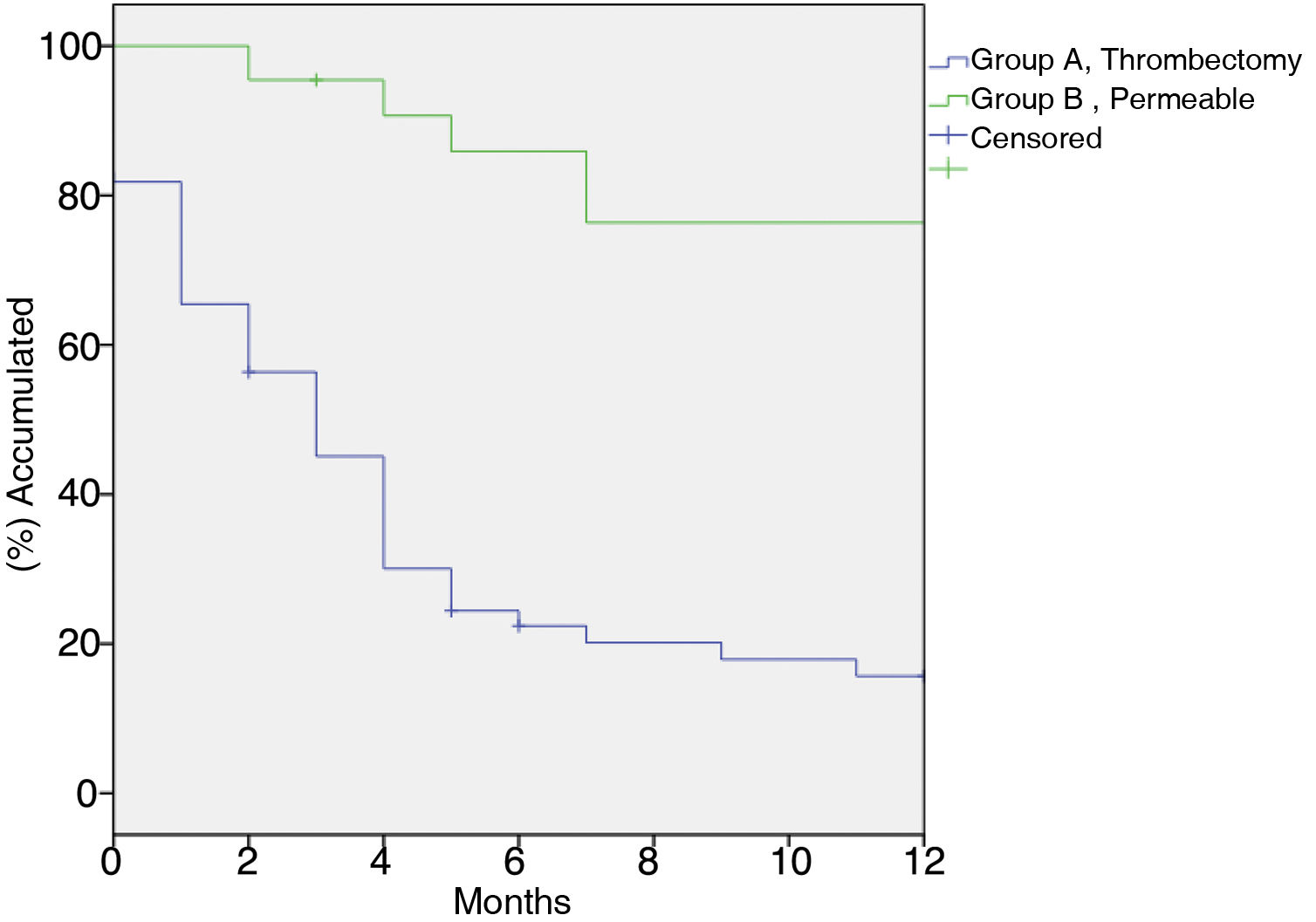
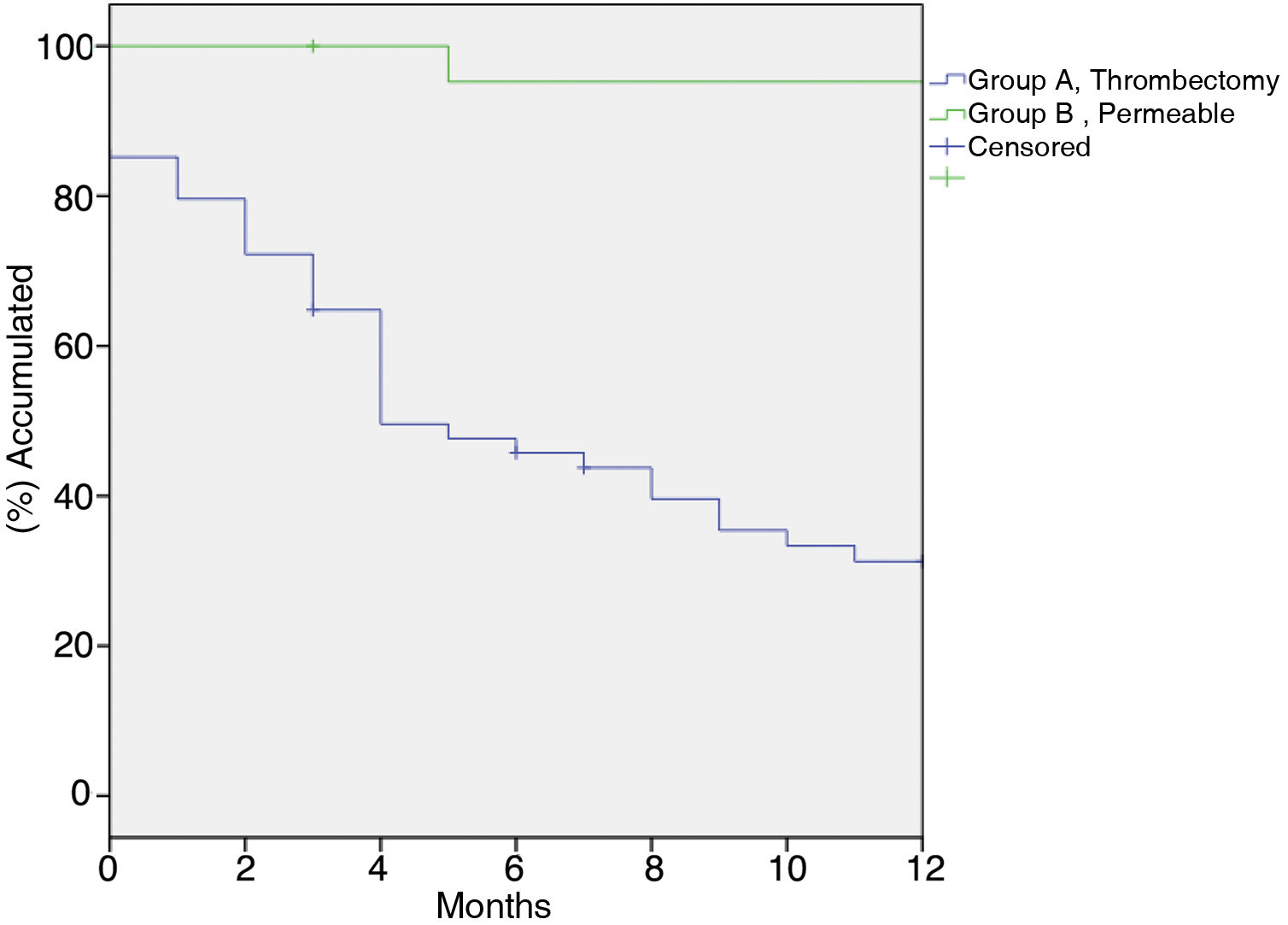
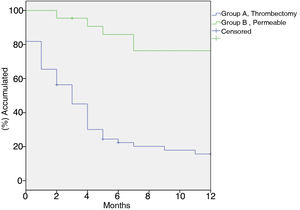
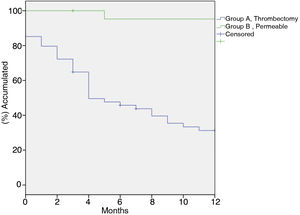
Primary patency at 1, 6 and 12 months was: Group A: 81.8%, 22.4% y 15.7% respectively. Group B: 100%, 85.9%, 76,4% (p < 0.001). Secondary patency at 1, 6 and 12 months was: Group A: 85.2%, 45.8% y 31.3% respectively. Group B 100%, 95.3%, 95.2% (p < 0.001). Use of non-covered stents was associated with an increased risk of occlusion (HR 2.669 IC 95% 1.146–6.216, p = 0.010).
ConclusionA higher patency of EV performed on a patent HAG is expected. It is therefore advisable to develop surveillance programs that are capable to detect VAS before its occlusion.
Analizar los resultados del tratamiento endovascular (TEV) de las estenosis en las anastomosis venosas (EAV) de las fístulas arteriovenosas protésicas (FAVp), comparando su utilidad al realizarse sobre FAVp permeables frente a trombosadas.
Material y MétodosEstudio retrospectivo de pacientes intervenidos mediante TEV por EAV de fístulas humeroaxilares realizadas entre enero de 2009 y diciembre de 2019 en nuestro centro. Grupo A: FAVp trombosada secundaria a EAV; Grupo B: FAVp permeable con EAV detectada en seguimiento. Se definió éxito técnico como estenosis residual ≤30% y éxito clínico como diálisis efectiva inmediata. Tras el TEV se realizó un seguimiento clínico y con Eco-Doppler semestral. Estudio estadístico: se realizó un análisis de supervivencia mediante el método Kaplan Meier para el estudio de permeabilidades.
ResultadosGrupo A: 55 pacientes. Grupo B: 22. No existieron diferencias significativas en las características demográficas ni anatómicas entre grupos.
El éxito técnico y clínico fueron del 100% en el grupo B frente a un 94,5% y 91% respectivamente en el grupo A.
La permeabilidad primaria a 1, 6 y 12 meses, en el Grupo A fue: 81,8%, 22,4% y 15,7% respectivamente, frente al Grupo B: 100%, 85,9%, 76,4% (p < 0,001). Permeabilidad secundaria a 1, 6 y 12 meses; en el Grupo A fue 85,2%, 45,8% y 31,3% respectivamente, frente al Grupo B 100%, 95,3%, 95,2% (p < 0,001). El uso de stents no cubierto se asoció a un mayor riesgo de oclusión (HR 2,669 IC 95% 1,146-6,216, p = 0,010).
ConclusiónEs esperable una mayor permeabilidad del TEV realizado sobre una FAVp permeable, por lo que es recomendable elaborar programas de seguimiento que sean capaces de detectar la EAV previo a la trombosis de esta.
Vascular access (VA) plays an essential role in the management of kidney patients, both because of the morbidity and mortality that it entails and because of its impact on the patient's quality of life1–4. That is why maintaining a functioning VA is a real challenge, both for patients and for the doctors.
Despite the fact that, due to their lower morbidity and greater permeability, autologous accesses are the best VA and most recommended for all patients5, a high percentage of patients perform dialysis through prosthetic fistulas made of polytetrafluoroethylene6,7 (PTFE). This access has a high rate of failure during the first 18 months after the placement7, mainly due to the development of intimal hyperplasia in the venous anastomosis8.
However, and despite the fact that the different scientific societies recommend follow-up for early detection of dysfunction9–12, it is controversial whether it should be performed a preventive repair because there are doubts about the benefits it provides in terms of patency or long-term durability of the fistula13,14.
Thus, the purpose of this study is to analyze the results of endovascular treatment (EVT) of venous anastomosis stenosis (VAS) in prosthetic arteriovenous fistula (pAVF), comparing its usefulness when performing permeable pAVF versus thrombosed pAVF.
MethodsWe retrospectively analyzed all the patients who underwent EVT in the Hospital Clínico San Carlos (HCSC) between January and December of 2019, to treat the stenosis in the venous anastomosis of the pAVF. Patients without follow up were excluded. The study was approved by the local ethics committee and informed consent was obtained from all patients included in the study.
Patients were separated into two groups according to the situation of the fistula at the time of treatment:
- none-
Group A: Thrombosed pAVF resulting from VAS (intraoperative detection).
- none-
Group B: Patients with VAS of pAVF.
The detection of VAS was based on the occurrence of difficulties with dialysis performance and/or ultrasound findings. The following combinations of findings will be used as criteria of severity or high risk of thrombosis:
- none-
Vascular lumen reduction >50% + peak systolic velocity (PSV) ratio >2.
- none-
Added to a flow rate Q A [mL/min] <600 or a flow reduction >25% as compared to the previous measurement.
All procedures were performed in an operating room with “C-arc” type X-ray equipment. In group B, an intraoperative fistulography confirmed the findings prior to treatment, while patients in group A were rescued by surgical thrombectomy and subsequent fistulography to assess patency.
Primary stenting was performed in all cases, using three types of devices: self-expanding stents, covered stents, or drug-eluting stents.
Technical success was defined as residual stenosis <30%, and clinical success was defined as immediate effective dialysis capable of reestablishing normal hydration, normal Blood Pressure, fluid and electrolyte balance and correction, at least partially, the metabolic and systemic abnormalities of uremic syndrome.
After surgery, all patients were scheduled every six months for clinical follow-up and Doppler ultrasound. In the event of problems occurring during the hemodialysis session patients were referred to our center.
Primary permeability was defined as the period elapsed from the performance of the procedure until thrombosis of the VA or the need for therapeutic or preventive procedures for the rescue of the VA. Secondary patency was considered as the time elapsed since the therapeutic intervention to the definitive thrombosis of the pAVF.
The demographic characteristics and comorbidity of the patients were collected. The preoperative anatomical characteristics of the artery and vein and intraoperative data were also recorded.
Statistical analysisThe primary objective of the study was to evaluate and compare primary and secondary patency in both groups. The secondary objective was to identify factors, both pre- and intraoperatively that could be associated with better patency. Variables are expressed in absolute numbers and percentages. The Kolmogorof-Smirnof test was used to evaluate the distribution of the quantitative variables. Continuous variables with normal distribution are expressed as mean and standard deviation, and variables without a normal distribution are presented as median and interquartile range. The Student t-test was used to evaluate the differences between the variables with normal distribution, and the Mann-Witney U test if they did show a normal distribution. Qualitative variables were compared using the Chi-Square test or Fisher's Exact test. Survival to an event was estimated using the Kaplan-Meier method and multivariate analysis was performed using Cox regression to detect other possible factors associated with increased survival.
The p value <0.05 was considered statistically significant. The analysis was performed using the statistical package SPSS (version 20.0; IBM Corporation, Somers, NY, USA).
ResultsA total of 204 pAVFs were created between January 2009 and December 2019 in our center, of which 91 were repaired due to VAS. Of these, 8 were treated using open surgery, and 6 did not have a subsequent follow-up, so they were excluded from the study, with a total of 77 patients finally included.
There were 55 cases that underwent treatment of thrombosis of the pAVF (71.4%, group A), as compared with 22 who had a patent pAVF at the time of surgery (28.6%, group B). The mean time elapsed from the placement of the VA until repair from completion was 9 (±8.63) months in group A, and 7.84 (±7.4) in group B, with no significant differences between groups (p = 0.602).
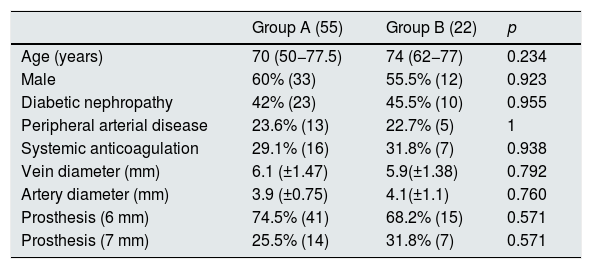
The preoperative anatomical characteristics and the demographic data are collected in Table 1. There were no significant differences in the cause of chronic renal failure or other comorbidities, as well as in the characteristics of the preoperative mapping or prosthesis used.
Demographic and preoperative variables.
| Group A (55) | Group B (22) | p | |
|---|---|---|---|
| Age (years) | 70 (50−77.5) | 74 (62−77) | 0.234 |
| Male | 60% (33) | 55.5% (12) | 0.923 |
| Diabetic nephropathy | 42% (23) | 45.5% (10) | 0.955 |
| Peripheral arterial disease | 23.6% (13) | 22.7% (5) | 1 |
| Systemic anticoagulation | 29.1% (16) | 31.8% (7) | 0.938 |
| Vein diameter (mm) | 6.1 (±1.47) | 5.9(±1.38) | 0.792 |
| Artery diameter (mm) | 3.9 (±0.75) | 4.1(±1.1) | 0.760 |
| Prosthesis (6 mm) | 74.5% (41) | 68.2% (15) | 0.571 |
| Prosthesis (7 mm) | 25.5% (14) | 31.8% (7) | 0.571 |
Technical success was 100% in group B and 94.5% in group A. In one case, repair was not considered after thrombectomy due to the characteristics of the lesion, and in the other two remaining cases a repair was not achieved because an adequate opening of the stent after its post-dilation was not accomplished due to long stenoses with marked deterioration of the prosthetic material.
Clinical success was 100% in group B, compared to 91% in group A, since five cases presented immediate occlusion after procedure or during the first hemodialysis session, without allowing clinically effective therapy.
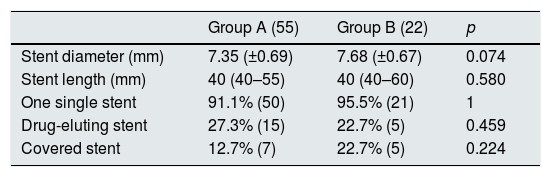
Regarding the type of device used, there were no significant differences between both groups (Table 2).
Twelve covered stents were implanted, 7 in group A (12.7% of the total) and 5 in group B (22.7%). The use of drug-eluting stent was 27.3% in group A and 22.7% in group B, and uncovered self-expanding stents were used in the rest of the cases. The median follow-up was 9 months (3–20).
In group A primary patency at 1, 6, and 12 months was 81.8%, 22.4%, and 15.7%, respectively; in group B primary patency was 100%, 85.9%, 76.4% (p < 0.001) (Fig. 1). Secondary patency at 1, 6, and 12 months in group A was 85.2%, 45.8%, and 31.3%, respectively, versus 100%, 95.2%, 95.2% in group B (p < 0.001) (Fig. 2).
There was no significant difference in the rate of reintervention between both groups (32.7% in group A vs. 36.4% in group B [p = 0.761]).
In the multivariable analysis, the uncovered stent was independently associated with lower patency as compared to endoprostheses (HR 2.669; 95% CI; 1.146–6.216, p = 0.010). No other association factors were observed.
DiscussionThrombosis is the most common complication of VA and the main cause of definitive loss of the fistula. This determines a series of negative consequences in the patient on renal replacement therapy; it increases morbidity and mortality, the frequency of hospitalization and healthcare costs15.
Achievement of patency of VA is not always possible but different authors have described greater patency in those rescued preventively prior to thrombosis16,17, in the latest guidelines there is no clear consensus regarding the usefulness of a follow-up9–12. For example, the latest GEMAV9, KDOQI10 and ERA-EDTA11 guidelines recommend, according to the usual concept of significant stenosis, monitoring pAVFs using first-generation and not second-generation methods, while the ESVS12 does recommend it.
The Spanish group of Gruss et al.6 published results that were similar to those shown in the present manuscript. They reported that the VA repaired after its occlusion presents a higher risk of definitive loss of the vascular access as compared to those treated for stenosis that were permeable (HR 4; 1.3–13.6).
Similarly, Sands et al.16 found in a series of 97 pAVFs a longer patency of those undergoing a preventive repair than those repaired after being thrombosed (1023 vs. 689 days, p = 0.01); similar results have been published by others17–21.
Regarding method of detection, Malik et al.22 published a controlled prospective study in which they demonstrated greater patency of pAVFs subjected to ultrasound monitoring. They compared clinical surveillance versus Doppler ultrasound, and they showed that the Doppler ultrasound allowed a greater detection of dysfunctional accesses and an increase in patency at one year.
Dember et al.13 conducted a clinical trial that included 64 patients randomized to an intervention group and the observation group. Although there were no significant differences in the time elapsed until definitive loss of the access, it is important to mention that in the observation group there was a higher rate of thrombosis (72% vs. 44%). Likewise, 8% of the thromboses in the intervention group occurred prior to treatment, and of the total number of accesses lost at the end of follow-up, only 16% were secondary to thrombosis, compared to 34% in the observation group.
Similar findings are reported by Ram et al.23, with a lower rate of definitive occlusion in the group treated prior to thrombosis, but without a more prolonged useful life of the pAVF. They do not specify the reason for abandonment and in their conclusions do not rule out the possible, albeit modest, benefit of preventive treatment.
In addition, it must be taken into account that, unlike the present study, both mentioned trials use simple angioplasty as rescue treatment, so the results must be interpreted with caution, since the superiority of stenting in this area seems to have been demonstrated24,25.
We have not found studies in the literature similar to the one presented here, so this is the first study to compare the results of the stent as a treatment method in this context.
An interesting finding to be highlighted is the higher rate of thrombosis observed in uncovered devices. Based on the concept that intimal hyperplasia is the pathophysiological process responsible for juxtanastomotic stenosis, it has been proposed the covered stent should be useful in the treatment of this pathology26–28. It is reasonable to assume that, excluding the endothelial tissue, the results are better than simple angioplasty or the uncovered stent, as our results seem to indicate.
The retrospective nature of the study has prevented us from having the functional data of group A prior to its inclusion in the study, which is an important limitation when it comes to knowing which pAVFs were previously dysfunctional and what is the appropriate method for their early detection.
ConclusionsThe EVT applied on a patent pAVF results in greater permeability than the EVT performed after its occlusion.
Despite the heterogeneity of the published studies and the lack of evidence regarding the usefulness of follow-up for early detection of VAE, our results suggest that it would be advisable to establish follow-up programs capable of detecting VAE prior to the thrombosis of pAVF.
Likewise, as compared to the use of uncoated devices, the use of covered stents could improve the results of the rescue of dysfunctional VA.
FinancingThis research has not received specific aid from public sector agencies, the commercial sector or non-profit entities.
Conflict of interestsThe authors declare that they have no conflict of interest.