Monoclonal gammopathy of renal significance includes all renal disorders caused by a monoclonal immunoglobulin secreted by a non-malignant B-cell clone. Patients with MGRS do not, by definition, meet criteria for multiple myeloma, with haematological disorders generally considered to be monoclonal gammopathy of undetermined significance. Nevertheless, the renal involvement can be serious and require specific treatment. Monoclonal gammopathy of renal significance is associated with a wide spectrum of disorders, including the recently discovered C3 glomerulopathy. Development of C3 glomerulopathy in the context of monoclonal gammopathy of renal significance after kidney transplantation is uncommon and very few cases have been published to date. We report on three cases of C3 glomerulopathy in the context of de novo monoclonal gammopathy after kidney transplantation.
La gammapatía monoclonal de significado renal incluye todas las enfermedades renales causadas por una inmunoglobulina monoclonal secretada por un clon de célula B no maligno. Por definición, los pacientes con gammapatía monoclonal de significado renal no cumplen criterios de mieloma múltiple y la alteración hematológica es generalmente considerada gammapatía monoclonal de significado incierto. No obstante, la dolencia que pueden causar a nivel renal puede ser importante, requiriendo un tratamiento específico. El espectro de la gammapatía monoclonal de significado renal es amplio, incluyendo una entidad reciente como la nefropatía C3. El desarrollo de una nefropatía C3 en el contexto de una gammapatía monoclonal de significado renal tras el trasplante renal no es frecuente y hasta el momento ha sido poco descrita. A continuación presentamos 3 casos de nefropatía C3 asociados a una gammapatía monoclonal de aparición de novo tras el trasplante renal.
Monoclonal gammopathy is the proliferation of the B lymphocyte cell line that produces a monoclonal immunoglobulin or its fragment (light chain or heavy chain). Different haematological conditions may produce a monoclonal gammopathy: monoclonal gammopathy of uncertain significance, multiple myeloma, plasmacytoma, Waldenström macroglobulinemia, chronic lymphatic leukaemia and lymphomas.1
Monoclonal gammopathy of uncertain significance consists of the presence of a monoclonal gammopathy without an associated malignancy but it may be the precursor of malignancies such as multiple myeloma or lymphoma. The progression rate to a malignancy is 1%/year.2 Monoclonal gammopathy of uncertain significance is a very frequent entity, occurring in a 3% of the population of more than 50 years of age.3 The diagnosis requires 3 criteria: monoclonal component inferior to 3g/dL, less than 10% of plasma cells in the bone marrow and no evidence of organic involvement.4
The monoclonal gammopathy by itself may produce different pathologies not related to the clonal mass but with characteristics of the monoclonal protein, this is called monoclonal gammopathy of clinical significance. The organs more frequently affected are: kidney, nervous system, skin, eye...5
The monoclonal gammopathy of renal significance (MGRS), includes all renal diseases caused by a monoclonal gammopathy (without associated neoplastic disease). The spectrum of MGRS is very broad and includes a recent entity such as C3 nephropathy.6–8 To date, there is limited information about the occurrence of this disease after kidney transplantation. Here we present 3 cases of de novo C3 nephropathy after renal transplantation in the context of a MGRS.
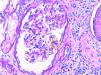
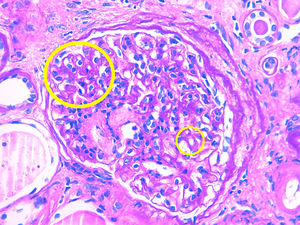
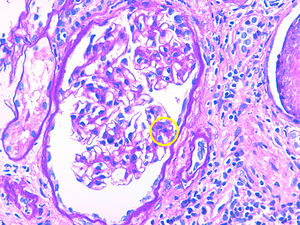
Description of casesCase 1A 66-year-old woman with chronic kidney disease stage 5 secondary to PKD due to mutation of the PKD1 gene. Hemodialysis was started at 48 years. Five years later, she received a first kidney transplant from a cadaveric donor, and induction immunosuppression was performed with prednisone, tacrolimus and mycophenolate mofetil. A correct function of the renal graft is achieved with a serum creatinine around 100–120μmol/L and without proteinuria. Seven years after renal transplantation (June 2012) there is evidence of proteinuria of 3g/day along with a slight deterioration of renal function. For this reason, a renal biopsy was performed showing global and diffuse mesangial and endocapillary proliferative glomerulonephritis, with negative immunofluorescence (IF). With this result, treatment with ACE inhibitors was started, stabilizing proteinuria and renal function for 2 years, subsequently there was a rapidly worsening renal function with increasing proteinuria up to 6g/day. A new renal biopsy was performed showing generalized endocapillary proliferative glomerulonephritis with an IFTA of 20%, IF positive for C3 in the loops and mesangium, leading to the diagnosis of C3 nephropathy. A proteinogram showed a monoclonal IgA lamda component (quantification: 3g/L) with negative PBJ and a normal ratio of light chains K/L in serum. The bone marrow aspirate shows 4% of plasma cells. Complement is normal and the genetic study of the complement does not show mutations. Presently the renal function is very deteriorated so no specific treatment is performed and requires maintenance dialysis.
Case 2A 75-year-old patient with chronic kidney disease stage 5 of unknown cause (without proteinuria). He started peritoneal dialysis at the age of 59. After 5 years, he received a first kidney transplant from a cadaveric donor, and induction immunosuppression was performed with thymoglobulin, prednisone, mycophenolate mofetil and delayed tacrolimus. Proper renal function is achieved with serum creatinine of 130–150μmol/L without proteinuria. After 6 years of the transplant, the patient presented proteinuria of 1g/day with stable renal function, so renal biopsy was performed. The biopsy demonstrates segmental and diffuse mesangial and endocapillary proliferative glomerulonephritis, and a definitive diagnosis cannot be established due to the lack of glomeruli to perform IF. Treatment with ACEI is started, achieving stabilization of proteinuria. Three years later proteinuria sharply increased to 5g/day and renal function was deteriorated to serum creatinine levels of 200μmol/L. Thus, a new renal biopsy was performed showing mesangial and endocapillary proliferative glomerulonephritis generalized with IFTA of 20% and the IF demonstrated mesangial and subendothelial C3 deposits, making the diagnosis of C3 nephropathy. The proteinogram presented monoclonal component IgM kappa (quantification: 2.5g/L) with negative PBJ and normal ratio K/L light chains in serum. The bone marrow aspirate showed 2% of plasma cells. The complement was normal and no mutations could be characterized but it was evidenced the presence of antifactor H antibodies. It was decided to perform treatment with rituximab (single dose of 1g) with good initial response, proteinuria and renal function was stabilized for a few months, but later renal function deteriorated requiring hemodialysis.
Case 3A 56-year-old man with chronic kidney disease stage 5 of unknown cause (without proteinuria). Hemodialysis started at the age of 49. After 6 years, he received a first kidney transplant from a cadaver donor, and induction immunosuppression was performed with thymoglobulin, prednisone, mycophenolate mofetil and delayed tacrolimus. Renal graft function improves to serum creatinine 160–170μmol/L without proteinuria. One year after transplantation, he presented a proteinuria of 1.5g/day; the renal biopsy showed acute borderline cell rejection with moderate IFTA. It was decided to maintain immunosuppression in the high range and treatment with ACEI was started, maintaining a stable proteinuria. Four years later, proteinuria increased very severely to 9g/day without nephrotic syndrome and renal function decreased rapidly to serum creatinine 300mg/L. A renal biopsy was performed showing focal and segmental mesangial and endocapillary proliferative glomerulonephritis with IFTA of 50 and a 30% of glomerular sclerosis. Congo Red staining is negative. IF showed gross granular deposit of C3 in loops and mesangium making the diagnosis of C3 nephropathy. The proteinogram showed monoclonal component IgG kappa (quantification: 1.6g/L) with a normal ratio of K/L light chains in serum and a bone marrow aspirate with 5% of plasma cells. The complement was normal and the genetic study was negative Due to important IFTA and glomerular sclerosis in the biopsy and since the renal function worsens rapidly, no specific treatment was performed and the patient was started again on regular hemodialysis (Table 1 and Figs. 1 and 2).
Relevant clinical data.
| Patient | Cause of CKD | Time TR-MGRS | MC | Specific Treatment | Time MGRS-DI |
|---|---|---|---|---|---|
| Case 1 | PKD | 10 years | IgA lambda | No | 4 months |
| Case 2 | Unknown | 9 years | IgM kappa | Rituximab | 15 months |
| Case 3 | Unknown | 6 years | IgG kappa | No | 2 months |
MC: type of monoclonal component; time MGRS-DI: time from the diagnosis of MGRS to the start of dialysis (years).
Time TR-MGRS: time of renal transplantation until the diagnosis of MGRS (years).
The monoclonal gammopathy of renal significance includes all forms of renal involvement caused by a monoclonal immunoglobulin secreted by a non-malignant B-cell clone (without associated neoplastic disease). Kidney involvement is diverse and may be very severe in some cases. It implies a high morbidity, which is why it is important to come out with an early diagnosis.6–8 Regarding the pathogenesis, kidney involvement may occur through two mechanisms. A direct mechanism, due to the deposit of the immunoglobulin or monoclonal immunoglobulin chain at the level of the glomerulus or the tubule. This would be the mechanism underlying the AL amyloidosis, the disease due to light chain deposits or the fibrillar and immunotactoid glomerulonephritis. The other mechanism is indirect, the monoclonal gammopathy produces a dysregulation of the alternative pathway of the complement causing a continued activation of this pathway. In this case, if the lesions are at the glomerular level it is a C3 nephropathy that includes C3 glomerulonephritis and the dense deposits disease; if the lesions affect the vessels it is an atypical HUS.6
Focusing on C3 nephropathy, the main diagnostic hypothesis is that the monoclonal protein would interfere with the proteins that regulate complement function, mainly the H factor. The monoclonal protein would act as an autoantibody against factor H, causing its inhibition causing a continuous activation of the alternative pathway of the complement that would produce glomerular damage.9
Both in C3 glomerulonephritis and in dense deposits disease, mesangial, membranoproliferative and endocapillary proliferation occurs with granular deposits of C3 at the glomerular level and the absence of immunoglobulin deposits. The difference between the 2 entities is observed by electron microscopy where in the case of C3 glomerulonephritis some granular deposit is observed at subendothelial, subepithelial or mesangial level and in dense deposits disease these deposits are seen in mesangium and infiltrating the glomerular basement membrane.10
The presentation of these pathologies is usually proteinuria. This may often progress to a nephrotic range. In the absence of specific treatment, there is a progressive deterioration of renal function and shortly after the patient requires renal replacement therapy. The diagnosis is made by renal biopsy, and it essential to perform IF and, if possible, the electronic microscopy.
There is little experience about the treatment of the MGRS, so it is not standardized yet. The treatment is based on the use of chemotherapy. In normal clinical practice, sometimes, it is difficult to encourage haematologists to perform this type of treatment in the absence of a basic neoplastic process. If the monoclonal component is IgM, the most accepted treatment at the present time is the use rituximab with or without cyclophosphamide. If the monoclonal component is IgA or IgG, it is advised the use of bortezomib associated or not to cyclophosphamide. However, it is evident that it is necessary to have more experience on these pathologies to establish treatment guidelines and to learn from the long-term prognosis of these entities on these treatments.6,8
From our experience, making an early diagnosis is essential in order to effectively apply a specific treatment. In cases 1 and 3, the renal function was already very deteriorated at the time of diagnosis, thus no specific treatment was prescribed. In case 2, the biopsy showed little fibrosis and given the fact that monoclonal component was IgM, it was decided use rituximab, achieving a partial but temporary response, probably due to its late application.
Regarding the possibility of a new transplant of patients who have lost the graft due to this pathology, it could be performed, but with a very close follow-up in the post-transplant, both due to the possibility of recurrence of the kidney disease and the possibility of progression to malignancy. of gammapathy.
ConclusionsThe monoclonal gammopathy of renal significance in the form of C3 nephropathy after kidney transplantation is narrowly described so far. It usually manifests in the form of nephrotic proteinuria with subsequent deterioration of renal function, which can lead to loss of the graft in a short time. In this context, it is very important to perform the diagnosis early in order to apply a specific treatment with chemotherapy at the initial phases of the disease. However, the most appropriate treatment guidelines for each specific clinical situation are still not well defined.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Serra N, Facundo C, Canal C, Arce Y, Ayasreh N, Vila A, et al. Tres casos de gammapatía monoclonal de significado renal postrasplante renal: nefropatía c3 de novo. Nefrologia. 2019;39:198–201.