A new cell-to-cell communication system was discovered in the 1990s, which involves the release of vesicles into the extracellular space. These vesicles shuttle bioactive particles, including proteins, mRNA, miRNA, metabolites, etc. This particular communication has been conserved throughout evolution, which explains why most cell types are capable of producing vesicles. Extracellular vesicles (EVs) are involved in the regulation of different physiological processes, as well as in the development and progression of several diseases. EVs have been widely studied over recent years, especially those produced by embryonic and adult stem cells, blood cells, immune system and nervous system cells, as well as tumour cells. EV analysis from bodily fluids has been used as a diagnostic tool for cancer and recently for different renal diseases. However, this review analyses the importance of EVs generated by stem cells, their function and possible clinical application in renal diseases and kidney transplantation.
En la década de los 90 se descubrió un nuevo sistema de comunicación célula-célula, que consiste en la liberación de vesículas cargadas con partículas bioactivas (proteínas, mRNA, miRNA, metabolitos, etc.) en el espacio extracelular. Este tipo de comunicación se ha conservado durante la evolución, hecho que justificaría que la mayoría de los tipos celulares puedan generarlas. Estas vesículas extracelulares (VE) pueden regular diversos procesos fisiológicos, así como el desarrollo y progresión de enfermedades. En los últimos años se ha extendido el estudio de las VE generadas principalmente por células madre adultas o embrionarias, células sanguíneas, células del sistema inmune y nervioso, así como células tumorales. El análisis de VE en fluidos corporales ha sido utilizado como herramienta de diagnóstico en cáncer y recientemente para distintas enfermedades renales. Sin embargo, en esta revisión pretendemos analizar la importancia, función y posible aplicación clínica de las VE generadas por células madre en enfermedades renales y en trasplantes.
The use of cell therapies to slow the progression of kidney diseases is a very promising approach due to the immunomodulatory and regenerative capacities of these therapies.1–5 The renal protection effect of mesenchymal stem cells (MSCs) is not only due to the capacity to transdifferentiate, but also to the impact of its activity on damaged tissue.1 Before using these therapies in routine clinical practice, there are a number of safety aspects that need to be investigated further: the possibility that a recipient's immune system rejection; the genetic cells stability; poor long-term differentiation; and the likelihood of virus transference.6–8 Therefore, it has been promoted the study of the mechanisms underling protective and regenerative capacity of stem cell therapy; the idea is to design alternative cell-free therapies. There are studies showing that MSC-secreted factors or MSC-conditioned media may have the same protective effect as MSCs on tissue damage and contribute to the immunomodulation of inflammatory states.9–13 The analysis of the conditioned medium evidenced the presence of growth factors, cytokines and extracellular vesicles (EVs). EVs may carry and transfer proteins, lipids and genetic material to resident cells in damaged tissue. EVs actively contribute to the therapeutic capacity of MSCs, in particular to the reprogramming of resident cells through the transference of mRNA and miRNA.9,14–25 Once demonstrated that EVs have the same therapeutic capacity as MSCs, EVs are being proposed as cell-free therapy being safer for patients.26
EV-mediated cell–cell communication is a mechanism that has been preserved throughout evolution in both eukaryotic cells and prokaryotic cells.27 Since its discovery 30 years ago,28 EVs have been shown to be produced by a large variety of cell types: blood, dendritic, endothelial and epithelial cells, as well as nervous system cells, adult and embryonic stem cells and even cancer cells.
EVs are formed by a lipid membrane and can transmit regulatory biological signals by transferring membrane and cytosolic proteins, lipids, mRNA, miRNA, mitochondrial DNA and genomic DNA that regulate various physiological processes, as well as in the development and progression of diseases.29–34 All cells can produce EVs as a normal mechanism of paracrine–endocrine communication; however in case of cell damage, EV production is increased and vesicular content is modified, to alert the adjacent cells, progenitor cells and the immune system. The body uses these processes to restore homeostasis in the damaged tissue. Only progenitor cells and MSCs can generate EVs with intrinsic protective or regenerative capacity.
As for the progression of diseases, it has been shown that the microenvironment defines the content of EVs. In arteriosclerosis in particular, vascular endothelial cells subjected to stress induced by calcium generate EVs that promote tissue mineralisation.35
Regarding cancer, it has been postulated that progenitor cells undergoing mutations, may be the origin of cancer stem cells,36 which produce EVs involved in the development and progression of cancer. These EVs promote angiogenesis,37 allow tumours to escape immune vigilance,38 induce the elimination of therapeutic molecules that activate apoptosis39 and actively participate in the degradation of the extracellular matrix required for metastasis.40 They act as paracrine–endocrine effectors by transporting bioactive molecules from cell to cell within the microenvironment, or by being remotely transported by body fluids.41
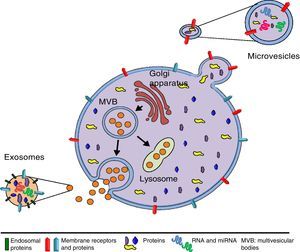
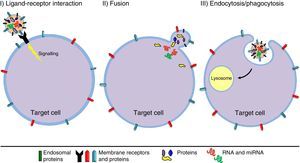
The origin and size of EVs allows us to differentiate between exosomes (EXs) and microvesicles (MVs) (Fig. 1). EXs are small vesicles (70–150nm) that are endosomal in origin; therefore their membranes are enriched with cholesterol, ceramides and sphingolipids, and their content corresponds to that which is present in the endosomal compartment. By contrast, MVs are larger (150–1000nm) and they are generated as a result of plasma membrane evaginations. The composition of MVs depends on the cell type they come from Refs. 42, 43 (Table 1). EVs can act through 3 mechanisms (Fig. 2): (I) by activating a signalling pathway of the target cell through adhesion with high specificity to the surface of the target cell (without membrane fusion), by the adhesion molecules and receptors on the cell surface.44 (II) By transferring mRNA, miRNA, proteins and signalling molecules, through membrane fusion.45 (III) By incorporating content through endocytosis in the target cells and processing their content in the endosomal compartment.46
Characteristics of extracellular vesicles.
| Exosomes | Microvesicles | |
|---|---|---|
| Size in nm | 70–150 | 150–1000 |
| Lipid composition | Low phosphatidylserine exposure. Lysophosphatidic acid. Cholesterol and ceramide | High phosphatidylserine exposure. Cholesterol |
| Protein markers | Alix, Tsg101, Hsc70, CD63, CD81, CD9 | Selectins, integrins, CD40, metalloproteinases |
| Origin | Multivesicular body | Plasma membrane |
| Secretion mechanisms | Exocytosis of multivesicular body | Evagination of the plasma membrane |
| Composition | Proteins, mRNA and miRNA | Proteins, mRNA and miRNA |
Presently, EVs are being used as urinary biomarkers of different kidney diseases and even for renal graft rejection.47–50 However, the purpose of this review is to analyse the importance, function and possible application of EVs in kidney diseases as an alternative therapy or as a therapy that complements the use of immunosuppressants in kidney transplantation. We will analyse different in vitro and in vivo models of kidney injury in which EVs from MSCs have been used to induced tissue regeneration.15,51–57
Acute renal failureAcute renal failure (ARF) is characterised by a loss, in hours or in days, of renal function resulting in, with an accumulation of creatinine and nitrogen metabolism products, such as urea. Although the causes remain controversial, the most common ones are ischaemia and reperfusion injury (I/R) or exposure to nephrotoxic agents that causes to acute tubular necrosis. The tubular injury induces the generation of inflammatory mediators that promote vasoconstriction and causes an inflammatory process accompanied by the infiltration of neutrophils which secrete reactive oxygen species, proteases and myeloperoxidases that aggravate kidney injury. ARF is considered one of the major causes of morbidity and mortality in hospitalised patients; therefore, there are myriad studies searching for biomarkers that allow an early diagnosis and investigating new therapies.1,58–60
The protective and regenerative effect of MSCs in animals models with ARF has been widely documented; in recent years, however, important evidence has emerged that shows that the protective effect is due to paracrine action and not to cell transdifferentiation.1,9,17–25 This paracrine effect includes proliferative, antiapoptotic and immunomodulatory effects. Specifically, in a damaged organ there is a microenvironment rich in cytokines, such as interferon gamma (IFNγ) and tumour necrosis factor alpha (TNFα); these cytokines stimulate MSCs and induce the secretion of different trophic and growth factors, cytokines and EV.61–63
The protective effect of EVs has been analysed in several ARF models in experimental animals: kidney injury induced by glycerol,15,16,64,65 cisplatin,54 gentamicin,55 I/R,52,66–70 unilateral obstruction of the ureter71 and 5/6 nephrectomy, 7 days post-surgery72 (Table 2). These ARF models can be characterised by tubular damage with a high inflammatory component associated with an increase in interstitial infiltrate, apoptosis and tubular necrosis. This microenvironment induces the selection of EVs in the same way as with MSCs. The accumulation of EVs in tissue could be promoted by the increased permeability of damaged tissues.64 The internalisation of EVs depends on the presence of cell receptors (CRCX4) and adhesion molecules (CD44 and CD29); the latter are found in both EV and MSC membranes.21 The most extensively studied EVs have been those generated from mesenchymal stem cells derived from human bone marrow (BM-MSCs). However, studies have also been conducted with EVs from other sources such as human liver stem cells; mesenchymal stem cells from umbilical cord blood; mesenchymal human cells from Wharton's jelly; circulating endothelial progenitor cells (EPCs); and, kidney-derived mesenchymal stem cells. All these EVs accelerate patients’ recovery from acute kidney injury in the same way that the producing cells do. This renoprotection can be characterised by an improvement in blood urea nitrogen and serum creatinine and also by an improvement in histological lesions.
Summary of animal models of acute kidney failure where EV therapy is applied.
| Model | Cellular origin of EVs | EV type | Route of administration | Therapeutic capacity | Reference |
|---|---|---|---|---|---|
| Glycerol | BM-MSC | MV | Intravenous | Morphological and functional recovery via mRNA and miRNA transfer | 16 |
| HLSC | MV | Intravenous | Decreased tubular necrosis and proliferation | 65 | |
| BM-MSC | MV | Intravenous | Morphological and functional recovery | 64 | |
| BM-MSC | MV | Intravenous | Induction of proliferation | 15 | |
| Ischaemia-reperfusion injury | EPC | EX | Intravenous | Decreased apoptotic and proinflammatory effect. Presence of proangiogenic transcripts | 70 |
| BM-MSC | MV | Intravenous | Induction of proliferation and inhibition of apoptosis. Proangiogenic effect | 68 | |
| WJ-MSC | MV | Intravenous | Induction of proliferation and inhibition of apoptosis. Anti-inflammatory effect | 69 | |
| UCB-MSC | MV | Intravenous | T-cell modulation | 67 | |
| EPC | MV | Intravenous | Induction of proliferation, inhibition of apoptosis and leucocyte infiltration. Proangiogenic effect | 66 | |
| BM-MSC | MV | Intravenous | Induction of proliferation, inhibition of apoptosis and leucocyte infiltration | 52 | |
| Cisplatin | UCB-MSC | EX | Injection into renal capsule | Decreased apoptosis, tubular cell necrosis and oxidative stress | 73 |
| BM-MSC | MV | Intravenous | Would improve renal function and survival | 54 | |
| Gentamicin | BM-MSC | EX | Intravenous | Induction of proliferation and inhibition of apoptosis and necrosis. Anti-inflammatory effect | 55 |
| Unilateral ureteral obstruction | BM-MSC | MV | Intravenous | Decreased lymphocytic infiltrate, tubular inflammation and necrosis | 71 |
| 5/6 Nephrectomy | BM-MSC | MV | Intravenous | Decreased fibrosis, interstitial lymphocytic infiltrate, and decreased or no tubular atrophy | 72 |
The mechanisms of action of EVs have been analysed in different in vitro models that include the use of renal tubular epithelial cells, endothelial cells and peripheral blood mononuclear cells. In all these cells, EVs have been shown to decrease the expression of inflammatory molecules, to stimulate cell proliferation and to inhibit apoptosis. Proteomic analysis of MVs derived from hUCB-MSCs showed the presence of proteins that have a protective effect on endothelial and tubular epithelial cells. These proteins include galectin-1 and galectin-3 which are mediators in the regulation of MSCs on T cells; 2 markers of MSCs, CD73 and CD90, associated with an immunosuppressive capacity; and, some elements of the complement pathway, such as CD59, C5, C3 and C4A. In addition, apolipoproteins, such as ApoA2, ApoA4 and ApoC3, that have been found to have a protective effect on the vascular endothelium in various pathological conditions, and lipid transport proteins, such as SCP2 and FABP6.67 This protein content can be radically modified if the cells receive a proinflammatory stimulus (IFNγ), which significantly reduces the protective capacity of MVs.67
Oxidative damage, characteristic of cisplatin-induced kidney disease, can be reduced by the action of hUCB-MSC EXs, which decrease the formation of oxidative products (e.g. 8-HdG and MDA) and increase GSH levels. EXs promote cell proliferation by activating ERK1/2.73
Acute kidney damage induces renal tubular cells apoptosis. To evaluate the impact of EVs on apoptosis, renal tubular epithelial cell cultures have been exposed to cisplatin. BM-MSC MVs halt the activation of genes related to cell cycle arrest, such as GADD45A, and apoptosis, such as Bcl-10, CASP-1, CASP-8, LTA, TP73 and CASP-10; and they decrease the gene expression of antiapoptotic agents, such as Bcl2, Bcl-XL, Akt1 and TRAF2. There is also an increase in the synthesis of renoprotective factors, such as hepatocyte growth factor and macrophage-stimulating protein. The mRNA of these factors is not part of the content of MVs, thus indicating that the activation of the metabolic pathways that triggers a regenerative phenotype must be induced by protein factors.15,54
In addition to the protein content, the genetic material (mRNA and miRNA) included in EVs can also modify the normal expression patterns of resident cells through horizontal transfer.74 In MVs derived from kidney mesenchymal stem cells, the presence of mRNA encoding vascular endothelial growth factor A (VEGF-A), type 1 insulin growth factor and the basic fibroblast growth factor stimulate endothelial cells and promote angiogenesis,68 whereas the study of EPC-derived MVs indicates that the presence of miR-126 and miR-296 are critical for stimulating angiogenesis. Transcriptome analysis of MSCs and their MVs has attributed relevance to the presence of transcripts related to cell differentiation, transcription control, proliferation and regulation of the immune system. Transcripts related to fatty acid oxidation, glycolysis, gluconeogenesis and the generation of ketone bodies have also been observed, and these are fundamental processes for the cytoprotection of renal tubular cells in processes such as ARF.16 Support for the importance of mRNA and miRNA on the protective effect of EVs has been shown by the use of different strategies: using ribonucleases to eliminate all mRNA and miRNA, specifically depleting miR-126 and miR-296 with antagonistic miRNA, or depleting all miRNA by eliminating the Dicer or Drosha genes, which are essential for the production and maturation of miRNA, in EV-generating cells.14–16
There are now several clinical trials (some in progress, some about to start) investigating the efficacy and safety of the application of MSCs in patients with acute renal failure (Table 3). However, the use of EVs to treat acute kidney diseases in humans has not yet started.
Stem cell clinical trials to treat acute kidney failure.
| Disease | Trial number | Title | Cell type | Current status |
|---|---|---|---|---|
| Acute renal failure | NCT01275612 | MSC in cisplatin-induced acute renal failure in patients with solid organ cancers (CIS/MSC08) | MSC | Screening |
| Acute renal failure | NCT00733876 | Allogeneic multipotent stromal cell treatment for acute kidney injury following cardiac surgery | BM-MSC | Completed |
| Acute renal failure | NCT01602328 | A study to evaluate the safety and efficacy of AC607 for the treatment of kidney injury in cardiac surgery subjects (ACT-AKI) | AC607 allogeneic BM-MSC | Finished |
| Renovascular hypertension | NCT02266394 | Hypoxia and inflammatory injury in human renovascular hypertension | MSC | Screening |
The increase in the prevalence of chronic kidney disease in the adult population makes it necessary to identify therapies that reverse the disease or that slow the progression to end-stage kidney disease.
MSCs have been used in different animal models of chronic kidney disease: models of reduced renal mass (5/6 nephrectomy),2,3,75–78 polycystic kidney disease,5 diabetic nephropathy,79,80 adriamycin-induced glomerulosclerosis,4 atherosclerotic stenosis81 or a model of cisplatin-induced chronic kidney disease.82 In the case of the 5/6 nephrectomy model, BM-MSCs improved renal function and reduced fibrosis in all the studies. This improvement is associated with a reduction in the progression of glomerulosclerosis2,3,75 and in the expression of interleukin-6 (IL-6) and TNFα, whereas expression of IL-4 and IL-10 is increased.76 Some other beneficial effects are also observed; there is reduction in the expression of vascular endothelial growth factor, p21 and proliferating cell nuclear antigen77 and the formation of new epithelium through the activation of Pax-1, a basic fibroblast growth factor, bone morphogenetic protein (BMP-7) and Tie-2 is also observed.78 In the polycystic kidney disease model in rats, BM-MSCs were shown to improve vascular density and therefore renal function.5 In several studies analysing diabetic nephropathy, the administration of BM-MSCs of murine or human origin were shown to reverse hyperglycaemia and streptozotocin-induced glycosuria.79,80 In the adriamycin-induced glomerulosclerosis model, MSCs can reach the damaged kidney and supply survival factors that preserve podocyte viability while reducing inflammation and glomerular sclerosis.4 In the model in which atherosclerotic stenosis was induced in the renal artery in pigs, there was chronic kidney damage characterised by extensive inflammation, apoptosis, oxidative stress, loss of microvasculature, fibrosis and glomerulosclerosis. Administration of MSCs from adipose tissue after a percutaneous transluminal renal angioplasty improves renal function.81 In the model of cisplatin induced chronic kidney disease in non-human primates, the preventive use of autologous BM-MSCs delayed the progression of interstitial fibrosis, but it could not reverse established damage.82
In clinical studies the administration of 3 doses of allogeneic BM-MSCs to a patient with recurrent focal and segmental glomerulosclerosis after kidney transplantation reduced the proteinuria and helped to maintain stable renal function and avoid conventional treatment with weekly plasmapheresis.83
All these findings, and many others, have provided sufficient information to begin conducting clinical trials to evaluate the efficacy and safety of MSCs (Table 4).
Stem cell clinical trials to treat chronic kidney disease.
| Disease | Trial number | Title | Cell type | Current status |
|---|---|---|---|---|
| Chronic kidney disease | NCT02195323 | (BM-MSC) in patients with chronic kidney disease (CKD) | Autologous BM-MSC | Complete |
| Chronic kidney disease | NCT01876017 | Safety and efficacy of BMMNC in patients with chronic renal failure | BM-mononuclear stem cell (BM-MNC) | Unknown |
| Chronic kidney disease | NCT01453816 | Study to assess the safety and effects of autologous adipose-derived stromal cells delivered in patients with renal failure | Autologous adipose-MSC | Unknown |
| Polycystic kidney disease-chronic kidney disease | NCT02166489 | Mesenchymal stem cells transplantation in patients with chronic renal failure due to polycystic kidney disease | MSC | Complete |
| Diabetic nephropathy-chronic kidney disease | NCT02585622 | Novel stromal cell therapy for diabetic kidney disease | Novel stromal cell | Screening has not yet begun |
In recent years, some groups have extensively demonstrated the positive effect of EVs in ARF models; however, their use in chronic models has been nil. The only studies to analyse the impact of EVs on chronic damage have been in models of follow-up after acute renal damage induced by ischaemia-reperfusion (I/R) where it has been observed that EVs produced by EPCs, or by BM-MSC, simulate the effect of MSCs and prevent the acute damage and also chronic damage induced by I/R.14,52 According to these studies, the micro-RNAs — miR-126 and miR-296 — are responsible for the favourable outcome of the EPC EVs,14 whereas the RNA content of the BM-MSC EVs is responsible for activating the target cells.52
End-stage renal disease: cell therapy and its derivativesThe progressive loss of renal function in advanced chronic kidney disease is associated with the activation of the immune system, marked by renal and systemic inflammation. This inflammation implies the activation of the complement system, monocytes, macrophages and chemokines.84 Transplantation is the best replacement therapy for survival in patients with end-stage kidney disease, but ischaemic injury is one of the causes of delayed graft function, and it has been associated with an increase in episodes of acute rejection and reduced long-term graft survival.85 The most significant risk factors for progressive loss of renal function in transplanted kidneys are I/R injury and proteinuria.86 Despite the new immunosuppressive strategies, the long-term outcome have not improved during the past decade, owing to the development of chronic graft dysfunction and to the mortality rate of patients with functioning grafts. This later problem is due primarily to cardiovascular causes and malignant neoplasms.87–91
Prolonged use of immunosuppressive therapy leads to side effects that are moderate but that in some cases contribute to the onset of more serious diseases, including diabetes and cancer, among others. Therefore, it is necessary to find alternative therapies that allow for the reduced use of immunosuppressants and that, ideally, induce graft tolerance. In this sense the use of MSCs or regulatory cells has been a very interesting starting point. The beneficial effect of cell therapies on kidney transplant, by preventing I/R-induced injury, interstitial fibrosis, tubular atrophy and acute rejection, has been shown in several studies using experimental animals.92,93
There are now several clinical trials testing the efficacy and safety of cell therapies in kidney transplantation, including regulatory cells and stem cells from different sources (Table 5).
Cell therapy clinical trials to treat kidney transplantation.
| Pathology | Trial number | Title | Cell type | Current status |
|---|---|---|---|---|
| Kidney transplant | NCT02085629 | Mreg (The ONE Study) | Donor M reg (Mreg_UKR) | Screening |
| Kidney transplant | NCT02129881 | TregUK (The ONE Study) | Autologous regulatory T Cell Product | Screening |
| Kidney transplant | NCT02371434 | nTreg Trial (The ONE Study) | Autologous CD4+CD25+FoxP3+ natural regulatory T cells | Inclusion by invitation |
| Kidney transplant | NCT02091232 | T-regulatory cells in kidney transplant recipients (The ONE Study) | T regulatory cell | Screening |
| Kidney transplant | NCT02244801 | darTreg (The ONE Study) | Donor-alloantigen-reactive regulatory T Cell | Screening |
| Kidney transplant | NCT02252055 | ATDC Trial (ONEatDC) | Autologous tolerogenic dendritic cells (ATDCs) | Screening |
| Kidney transplant | NCT01446484 | Treatment of children with kidney transplants by injection of CD4+CD25+FoxP3+ T cells to prevent organ rejection | CD4+CD25+CD127low FoxP3+ T regulatory cells injection | Unknown |
| Kidney transplant | NCT02560220 | MIC cell therapy for individualised immunosuppression in living donor kidney transplant recipients | Mitomycin C-induced PBMC | Screening |
| Kidney transplant | NCT02057965 | Mesenchymal stromal cell therapy in renal recipients | Autologous BM-MSC | Screening |
| Kidney transplant | NCT02176434 | Pilot feasibility study of combined kidney and hematopoietic stem cell transplantation to cure end-stage renal disease | Hematopoietic stem cell | Screening |
| Kidney or liver transplant | NCT01429038 | Infusion of third-party mesenchymal stem cells after renal or liver transplantation. A phase I–II, open-label, clinical study | Third party MSC | Screening |
| Kidney transplant | NCT00658073 | Induction therapy with autologous mesenchymal stem cells for kidney allografts | Autologous MSC | Complete |
| Kidney transplant | NCT02387151 | Neptune | Allogeneic MSC | Screening |
| Kidney transplant | NCT02565459 | MSC in renal recipients to induce tolerance in recipients of kidney transplants from deceased donors | Third-party BM-MSC | Screening |
| Tolerance | NCT00183248 | Pilot study using donor stem cells and campath-1H to induce renal transplant tolerance (ITN022ST) | Donor BM-stem cells | Complete |
| Tolerance | NCT00752479 | MSC and kidney transplant tolerance | MSC under basiliximab/low dose RATG | Finished |
| Tolerance | NCT02012153 | aMSC to induce tolerance in living-donor kidney transplant recipients | Autologous MSC | Screening |
| Chronic rejection | NCT02563340 | Effect of BM-MSC on chronic AMR after kidney transplantation | BM-MSC | Screening has not yet begun |
| Delayed function | NCT02563366 | Effect of BM-MSC on early graft function recovery after DCD kidney transplant | BM-MSC | Screening has not yet begun |
| Delayed function | NCT02561767 | Effect of BM-MSC in DCD kidney transplantation | BM-MSC | Screening has not yet begun |
The use of EVs in the experimental kidney-transplant models is anecdotal so far. There is only one study that analysed the role of isogenic EVs produced by BM-MSCs, which reduced innate response by reducing natural killer cells infiltrating the graft, as well as a reduction in TNFα; however, they failed to suppress the adaptative immune response.94 In this respect, the research grant awarded to us by the Spanish Society of Nephrology in 2014 will allow us to analyse the impact of isogenic and allogeneic BM-MSCs, as well as their EVs, in a kidney transplant model in rats.
Limitations of the use of extracellular vesicles in clinical practiceAny therapy based on human EVs must be considered a biological medicinal product because it contains one or more active substances made or derived from a living cell, and it must be subject to the necessary regulations in Europe, the United States of America, Australia and Japan. These EVs must comply with the safety standards for tissues and cells because they have, in common with the source, complexity, composition and biological action. However, taking into account the relevance of the results obtained from animal models, it is assumed that therapies based on EVs are not included in the definition of high-risk investigational new drugs. To produce such a therapy, it is necessary to have infrastructure, technology and a quality-management system, to comply with GMP and GLP standards so the donor and recipient safety is preserved. During the initial phases of clinical studies, the safety, toxicity and immunogenicity of such a therapy must be monitored. More advanced clinical studies (phases II-IV) will evaluate the efficacy and adverse effects of autogenous or allogeneic EVs that will support the translation of this therapy to the clinical practice.95
Although the use of EVs produced by progenitor cells or MSCs has achieved a degree of success in acute preclinical models, there are still some unanswered questions that will have to be addressed before introducing therapies derived from these observations in the hospital setting. Which component or combinational of compounds form EVs is responsible for the regenerative effect? How does the biodistribution of EVs and their trophism towards tubular epithelial cells take place? Regarding the production of EVs the main limitations are the reduced secretion by producing cells, the complex characterisation of the content and presentation of HLA-surface antigens. Recognising allo-HLA may be a major drawback to EV therapy, but strategies are being sought to generate EVs without HLA or to synthesise them. The synthetic production of EVs with a content adjusted to the needs (miRNA, mRNA, proteins) that are not recognised by patients’ immune systems will be the final goal.
Development of synthetic vesicles for clinical useThe production of artificial or synthetic EVs goes alone with the use of liposomes, microparticles or nanoparticles composed of biodegradable polymers such as poly(lactic-co-glycolic acid) (PLGA),96–98 or collagen,99,100 or dextran.101–103 Synthetic vesicles will enable us to have a better control the release of compounds and the targeting of damaged tissues; to reduce side effects; and to increase bioavailability to ultimately augment the patients’ quality of life.104 In particular, liposomes are the most widely accepted system because of their biocompatibility and biodegradation, high solubility, increased half-life, selective release at the site of action and ability to resist the action of chemotherapeutic agents. Up to now, however, liposomes have shown a low specificity, although there are studies that have developed liposomes sensitive to temperature, pH, light, electric or magnetic fields, and able to couple with ligands and antibodies to their membranes.105
The large-scale production of liposomes and their formulation for clinical use also has several drawbacks: instability, toxicity following repeated administrations and complement activation.106,107 Despite these drawbacks, several liposome-based drug-delivery systems have been developed that are in the preclinical stage, clinical trials and some approved as a clinical treatment, specifically in the field of chemotherapy.108,109 Despite all these advances, the design of new liposomal formulations requires profound in vitro and in vivo research in order to carry out preclinical studies before they can be transferred to clinical use.
Our group, together with 3 European centres, is currently involved in the EV Stem Injury project, funded by the “FP7-PEOPLE-2013-IAPP - Marie Curie Action: Industry-Academia Partnerships and Pathways” programme. This project includes 2 different approaches to EV production: biological and synthetic. In order to explore the renoprotective effect of EVs, their potency and efficacy will be evaluated in in vitro and in vivo models of acute and chronic kidney injury.
Key concepts- •
Extracellular vesicles are a system of communication between cells that involves the transfer of protein and genetic material.
- •
There are different types of extracellular vesicles according to their origin and size.
- •
Progenitor stem cells and their extracellular vesicles feature the same therapeutic potential.
- •
The use of extracellular vesicles would prevent the risk of poor differentiation involving the use of undifferentiated stem cells.
- •
The renoprotective potential of extracellular vesicles has been extensively studied in acute models, but it is necessary to deepen their application in chronic models and kidney transplantation.
- •
The analysis of the extracellular vesicles content is essential in order to specify the component or cocktail of components with a renoprotective effect.
- •
The manufacture of synthetic vesicles with a more controlled composition and a therapeutic capacity equivalent to biological ones will make their future application possible in daily clinical practice.
The authors of this article declare that there is no conflict of interest.
LENIT's researchers are part of REDinREN (RD12/0021/0028) at Instituto de Salud Carlos III-Ministry of Science and Innovation, co-financed by the European Regional Development Fund (ERDF), “A way to make Europe”.
J.M.C. received support in the research done by S.E.N. in 2014.
This work was carried out at the Centre de Recerca Biomèdica Cellex [Cellex Biomedical Research Centre], Barcelona, Spain.
Please cite this article as: Rovira J, Diekmann F, Campistol JM, Ramírez-Bajo MJ. Uso terapéutico de las vesículas extracelulares en insuficiencia renal aguda y crónica. Nefrologia. 2017;37:126–137.