La cirrosis representa un estadio avanzado de la fibrosis hepática y conlleva a una alta morbimortalidad cuya complicación más frecuente es la ascitis. Una minoría de pacientes con cirrosis avanzada tiene «ascitis refractaria» y no responden al tratamiento convencional. La paracentesis evacuadoras de repetición se consideran el tratamiento de elección en estos casos. Una gran parte de estos pacientes presentan asociada una enfermedad renal crónica (ERC), que puede precisar de tratamiento renal sustitutivo (TRS). Debido a las complicaciones asociadas a la enfermedad hepática de alteraciones de la coagulación y tendencia espontánea a la hipotensión arterial plantea problemas de cara al TRS, especialmente derivados de la hemodiálisis (HD). En este sentido la diálisis peritoneal (DP) ofrece varias ventajas respecto a la HD en pacientes con cirrosis, con o sin ascitis debido a su mejor tolerancia hemodinámica por ser un técnica continua y lenta, con baja tasa de complicaciones infecciosas y hemorrágicas.
Cirrhosis represents a late stage of hepatic fibrosis and leads to high morbidity and mortality, and the most frequent complication is ascites. Only a few patients with advanced cirrhosis have 'refractory ascites' and do not respond to conventional treatment. Repeated paracentesis for evacuation is considered the treatment of choice in these cases. A large proportion of these patients have associated chronic kidney disease (CKD), which may require renal replacement therapy (RRT). Due to the complications associated with liver disease with coagulation disorders and tendencies towards spontaneous hypotension, there are significant problems associated to RRT, especially haemodialysis (HD). On the contrary, peritoneal dialysis (PD) offers several advantages over HD in cirrhotic patients (with or without ascites) thanks to better haemodynamic tolerance, as it is a continuous and slow technique. Furthermore, PD has a low rate of infection and bleeding.
INTRODUCTION
Liver disease, including cirrhosis, is one of the primary causes of human morbidity and mortality, and is the third leading cause among the population of adults aged 40-59 years.
Cirrhosis is an advanced state of progressive hepatic fibrosis characterised by altered liver structure and the formation of regenerative nodules. It is irreversible in advanced stages, and the only treatment option in these cases is liver transplant. Patients with cirrhosis are susceptible to a number of complications that determine a lower life expectancy. The most common complication is ascites. Cirrhosis and liver disease were the cause of over 25 000 deaths and 373 000 hospitalisations in the United States in 1998, according to a report by the National Centre for Health Statistics.1-8
ASCITES
Definition
Ascites is the pathological accumulation of liquid in the peritoneal cavity, and is brought on by portal hypertension. Ascites is the primary complication in cirrhosis. Its development is the final consequence of a series of anatomical (circulatory and vascular), functional, and biochemical disorders that cause abnormal liquid retention.9-11
Clinical manifestations and diagnosis
Generally, patients discover that they have ascites by observing an increase in abdominal circumference. When the accumulated liquid volume exceeds 500ml, ascites can be observed through a physical examination in the form of shifting dullness, fluid wave, and distension.
Physiopathology
Several different theories have been proposed to explain the pathogenesis of ascites, but the most recent and widely accepted theory is that of arterial vasodilation.12 This causes reduced peripheral vascular resistance and blood pressure, increased cardiac output, and consequently, hyperdynamic circulation. The secondary effects are activation of endogenous vasoconstrictors and water and sodium retention, which causes abnormal accumulation of liquid in the peritoneum.13-15 The increased synthesis of vasodilators such as nitric oxide has been recently involved in the pathogenesis of cirrhosis.16,17
Classification
A new classification system has been proposed by the International Ascites Club,18 involving the following grades:
Grade 1. Mild ascites only detectable by imaging tests (ultrasound).
Grade 2. Moderate ascites manifesting as moderate symmetrical abdominal distension.
Grade 3. Severe ascites, with notable abdominal distension.
However, the validity of this classification system has not yet been established, and the ascites classification system that ranges between 1+ and 4+ continues to be used, with 1+ being undetectable, minimal ascites, 2+ is moderate ascites, 3+ is massive but not tense, and 4+ is massive and tense.19
Prognosis
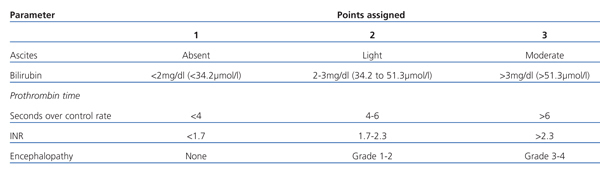
The prognosis in cirrhosis varies widely due to the number of factors involved, including aetiology, severity, and the presence of complications and associated comorbidity. The most useful method for stratifying the severity of the disease, surgical risk, and the general prognosis is by using the Child-Pugh classification system (Table 1).
A total score of 5-6 is considered grade A (well-compensated disease); 7-9 is grade B (significant functional compromise), and 10-15 is grade C (decompensated disease). These grades correspond to patient survival rates at 1 and 2 years, respectively: grade A: 100% and 85%; B: 80% and 60%, and grade C: 45% and 35%.
Treatment
The aim of treatment in patients with cirrhosis and ascites is to correct the underlying cause of the liver disease to the extent possible and to minimise water and sodium retention. The treatment objective is weight loss of 1kg/day (maximum), in the presence of ascites and peripheral oedema, or 0.5kg/day if the patient only has ascites. The most important component of patient treatment is the restriction of salt uptake. A diet of 800mg sodium (2g NaCl) is usually sufficient to induce a negative sodium balance and facilitate diuresis. If the sodium restriction is insufficient for increasing diuresis and inducing weight loss, diuretics must be prescribed, usually spironolactone, and a proximally acting diuretic can be added if the patient does not achieve adequate diuresis. However, 10% of patients are resistant to normal treatment measures. In these cases of resistant ascites, other therapeutic options are necessary.20,21
In patients with severe ascites, evacuation paracentesis is the most effective treatment. In some patients with ascites refractory to paracentesis, a latero-lateral porto-caval shunt can improve the condition, although these patients generally have very high surgical risk. The use of this technique is limited by the high frequency of complications, such as infection, disseminated intravascular coagulation, and shunt thrombosis. More recently, transjugular intrahepatic portosystemic shunts (TIPS) have provided effective control of ascites refractory to other treatments, although portal decompression upon mobilising the ascitic fluid has triggered severe hepatic encephalopathy in some patients.
PREVALENCE OF ASCITES IN CHRONIC KIDNEY DISEASE
The incidence of ascites in advanced chronic kidney disease (CKD) varies, but tends to fall between 0.7% and 20%.22 The prevalence of CKD along with hepatic cirrhosis and ascites is not precisely established, but there is a clear increase in the frequency of the occurrence of this association due to the growing prevalence of both diseases. Chronic liver disease frequently progresses along with renal disorders that lead to CKD, even reaching levels requiring dialysis treatment.23 The optimal time for commencing dialysis in these patients is difficult to determine, since they share symptoms such as anorexia and weight loss, among others, which could be due to both uraemia and liver disease. Additionally, the over-estimation of renal glomerular filtration rates leads the physician to attribute the symptoms to the liver disease more than to uraemia.24
Renal replacement therapy in patients with CKD associated with liver disease and ascites
No clinical trials have yet been carried out that accurately evaluate the impact of the different dialysis options available to patients with CKD and cirrhosis. The causes of ascites in patients on dialysis are: coexisting liver disease, coexisting cardiovascular disease, peritonitis, severe protein depletion, and idiopathic ascites. All of these situations represent a challenge to maintaining haemodynamic stability in the presence of CKD and dialysis is necessary, especially during haemodialysis sessions (HD).22
The primary limitation to the use of HD in cirrhotic patients is intra-dialysis hypotension. Patients with hepatic cirrhosis and ascites have reduced peripheral intravascular resistance. Under these circumstances, the sudden decrease in intravascular volume during the ultrafiltration (UF) process of HD frequently produces haemodynamic intolerance by exacerbating the level of hypotension. An added factor that can contribute to the instability of cirrhotic patients during HD is the increased production of nitric oxide during dialysis which has been demonstrated in patients without cirrhosis and with intra-dialysis hypotension.
In addition, there is also an increased risk of haemorrhage due to thrombocytopaenia and prolonged coagulation time, as well as gastrointestinal bleeding due to oesophageal varices or hypertensive gastropathy. The former limits the prescription of heparin during HD, but this natural elongation of coagulation times may be sufficient for avoiding trans-dialysis coagulation problems. Anticoagulation-free circuits can be used in these cases. Moreover, since no studies have evaluated the use of anticoagulants in cirrhotic patients, the exposure of patients to anticoagulants must be limited to the extent possible.
The assessment of dialysis adequacy in patients with severe ascites is under debate. Measuring the urea reduction ratio (URR) before balancing the urea levels of the large extracellular reservoir overestimates the dialysis dosage used, and so it is recommended to measure the equilibrated fractional clearance index of urea 1-2 hours after dialysis in ascites patients.
Another inconvenience of intermittent HD is the sharp changes in osmolarity and electrolyte levels that produce severe alterations in cerebral water levels, with consequent increased risk for developing encephalopathy. Continuous treatment with PD offers significant advantages in several different aspects to chronic hepatopathy/ascites patients, and even allows for partial and progressive draining of the ascitic fluid.23,24
Transmission of hepatitis C and B viruses
The prevalence of the hepatitis B virus (HBV) in dialysis centres varies widely, between 0% and 51%, and can be 8-9 times higher than in patients on peritoneal dialysis (PD).25 Nosocomial transmission is a risk factor for HBV infection of patients on HD. In contrast with other viral infections, the viral load in the blood can be increased in seropositive patients, and the HBV can survive in an open environment, which is a fact that must be taken into account in dialysis patients, above all those undergoing HD, since these can be at risk for a nosocomial HBV infection. Although it is known that the DNA of the HBV crosses the membrane of a dialyser during high-flow dialysis, the level of ineffectiveness of the dialysate and ultrafiltration is under debate.26,27 A reduced risk of being infected by HBV has been observed in patients on PD, with a 19 times lower seroconversion rate.28
In comparison, liver disease caused by the hepatitis C virus (HCV) is a significant cause of morbidity and mortality in dialysis patients. Additionally, these patients have a higher risk of contracting HCV than the general population. Several different factors have been identified: the number of transfusions, the duration of CKD (some studies have shown higher probability of infection after one decade of HD), the prevalence of HCV at the dialysis unit, and previous history of organ transplant or intravenous drug abuse. As such, patients treated in HD units with a high prevalence of HCV infection have a greater risk of being infected.29
The type of dialysis performed also seems to influence the risk of infection by HCV, with lower rates in PD than HD. Such was the case in a study from 2009 in which all incident dialysis patients were included from the registries of 10 different countries/regions of Asia and the Pacific (Australia, New Zealand, Japan, China, Taiwan, Korea, Thailand, Hong-Kong, Malaysia, and India) between April 1995 and December 2005.30 The seroprevalence rates for HCV were generally higher in patients on HD than PD (7.9% compared to 3.0%), with similar results in seroconversion rates (incidence rate ratios of 0.33 PD vs HD; 95% confidence interval [CI]: 0.13-0.75). Regarding HBV, the available data from this study were more limited (only 7 countries were included), although the authors did observe that the result were less influenced by the type of dialysis used.25
In another study involving 129 anti-HCV negative patients that were undergoing chronic dialysis treatment, the seroconversion rate for patients on HD was 0.15/patient-year as compared to 0.03/patient-year in PD patients.28 In addition, the majority of the patients on PD that tested positive for anti-HCV had acquired the disease while on HD. In accordance with these results, an Israeli study observed that the prevalence of HCV infection among HD patients was 18% and only 7% among PD patients.31
Several different factors explain the lower risk of HCV infection among PD patients: this technique requires fewer blood transfusions and absence of vascular access points and extracorporeal blood circuits, which reduces the risk of parenteral exposure to the virus during this outpatient procedure.29,32
PERITONEAL DIALYSIS AS A RENAL REPLACEMENT THERAPY ALTERNATIVE (Table 2)
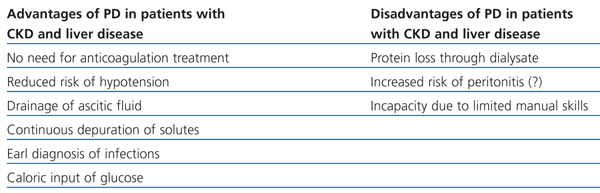
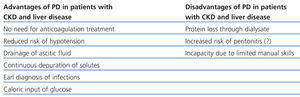
Patients with CKD and liver disease with ascites benefit from PD through several different mechanisms: improved haemodynamic tolerance with fewer episodes of hypotension, dialysis-ascites fluid drainage and early diagnosis of infection, avoiding the use of heparin, reducing the risk of haemorrhage and anaemia, better preservation of residual renal function, complementary glucose input, and reduced risk of contracting hepatitis in the case of positive patients.23
However, the fear of excessive bleeding during the placement of the catheter, inadequate ultrafiltration (UF) and removal of solutes in the presence of ascites, and the increased risk of bacterial peritonitis and hypoalbuminaemia have all limited its use.23,24
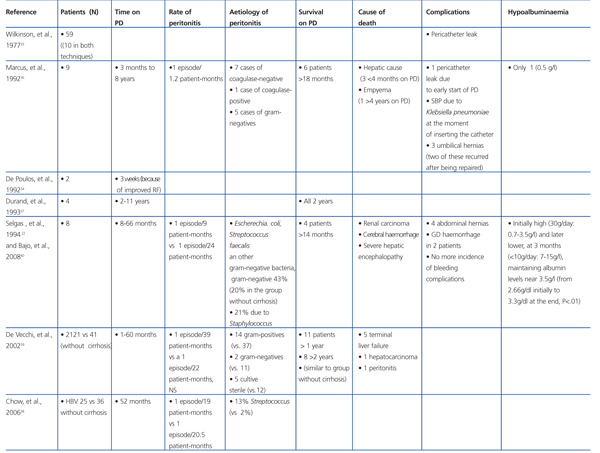
Paracentesis is considered to be the first line of treatment for severe ascites. Given that treatment is continuous, PD is a slow and continuous method of dialysis and water loss that imitates a daily regimen of paracentesis, and provides an alternative treatment for these patients, even for prolonged periods of time. As such, cirrhotic patients with temporary or chronic renal failure could benefit from the placement of a peritoneal catheter in order to repeatedly drain the ascitic fluid at home.22 Wilcox et al33 used PD catheters successfully for this purpose, with a mean drainage of 7.6 litres in 129 minutes.
Placement of the peritoneal catheter
The peritoneal catheter can be inserted percutaneously or surgically. Neither bleeding complications nor intestinal perforations have been reported to occur more often in this type of patient.22
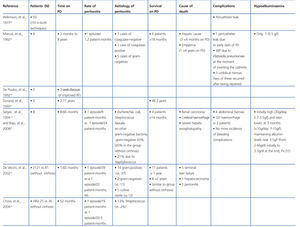
Marcus et al36 described five patients with prolonged coagulation times who underwent percutaneous catheter placement with no bleeding complications.
Peritoneal transport and adequacy
Patients with ascites have been observed to develop increased diffusion of urea, creatinine, phosphorous, and CO2 molecules through the peritoneal membrane, as well as increased UF capacity. The increase in UF is not correlated with glucose absorption (D/D0) after 4 hours, or with the concentration of sodium in the dialysis effluent (PD Na), probably due to the production of ascites. The increased UF capacity makes the use of hypertonic solutions especially important.22,24
Durand et al37 described the functional behaviour of the peritoneum in four patients, observing an initial increase in UF capacity and high solute clearance. Selgas et al22 and Durand et al37 described a higher UF capacity in cirrhotic patients than in non-cirrhotic patients.
Nutritional status and peritoneal protein loss
One of the problems associated with the loss of proteins through the effluent produced in these patients is the risk of malnutrition. However, in the study of cirrhotic patients and ascites treated with PD described by Selgas et al,22 these authors observed an initial loss of proteins in the peritoneal membrane at the start of dialysis treatment as high as 30g per day, but this loss later decreased to a mean of 7-15g/day. This effect was observed during the first three months of dialysis treatment; later, the reduced protein loss was correlated with increased serum albumin levels and the patient’s recovered body weight.22
Peritonitis
There are some discrepancies regarding the higher rate of peritonitis associated with cirrhotic patients with ascites compared to the rate of peritonitis in patients without cirrhosis. Chow et al38 found no differences in the incidence of episodes of peritonitis between patients with HBV and cirrhosis and those without cirrhosis (1 episode/19 patient-months in the group with cirrhosis compared to 1 episode/20.5 patient-months). Similarly, De Vecchi et al39 observed a similar incidence of peritonitis in cirrhotic and non-cirrhotic patients treated with PD (1 episode/39 patient-months compared to 1 episode/22 patient-months, NS). However, Selgas et al observed a higher incidence of peritonitis in patients with cirrhosis treated with PD than in non-cirrhotic patients (1 episode/9 patient-months compared to 1 episode/24 patient-months). The aetiology associated with peritonitis in patients with cirrhosis treated with PD varies. De Vecchi et al39 observed that the majority of the isolated microorganisms were gram-positive, primarily Staphylococcus (14 episodes), and only two episodes were caused by gram-negative bacteria. This result differs from other studies in which the more frequent cause was gram-negative bacteria.22
Given that spontaneous bacterial peritonitis (SBP), the pathology most frequently associated with ascites, is primarily caused by gram-negative bacteria, it is difficult to differentiate the infectious episodes that are due to the dialysis technique used from those secondary to the liver disease itself. In any case, continuous visualisation of the peritoneal liquid through a daily drain allows for an earlier diagnosis based on the turbidity of the dialysate and avoids the need for paracentesis in the case of suspected SBP. Additionally, having access to a peritoneal catheter, these patients could benefit from the intraperitoneal administration of antibiotics for the treatment of peritonitis.
Survival of the cirrhotic patient on peritoneal dialysis
In the study carried out by Marcus et al36 involving 9 patients with hepatic cirrhosis on PD, five survived longer than 18 months, two for two years, one for four years, and another for eight years. Bajo et al40 described six patients with cirrhosis and ascites treated with PD, and with a follow-up period of 8-66 months and good control. Three patients died due to causes unrelated to the technique used. Durand et al37 described four patients, three of which survived longer than 2 years.
The most recent and largest study was that performed by De Vecchi et al39 involving 21 cirrhotic patients on PD, and comparing them with a historical cohort of patients on PD and without cirrhosis. Of the 21 patients, 11 had been on PD for more than one year and 8 patients more than 2 years. There were no differences in mortality as compared to the PD group without cirrhosis.
In summary, the treatment of patients with CKD and chronic liver disease with ascites is a complex undertaking due to several different associated issues, including ascites and other complications derived from liver disease. Given the elevated morbidity and mortality rates derived from cirrhosis, special care must be taken when indicating and starting RRT in this population, especially with regard to the potential risks associated with HD. PD provides a viable alternative with several potential benefits, such as improved haemodynamic stability and reduced risk of bleeding. The theoretically higher risk of peritonitis has not been clearly shown by the medical literature, and publications tend to show a similar rate of peritonitis between patients undergoing PD with and without cirrhosis. Additionally, PD can facilitate proper solute clearance while allowing for relief from ascites symptoms. As such, in spite of the scarce clinical observations yet published, PD can be considered as a viable and effective dialysis technique for this group of patients.
KEY CONCEPTS
1. The prevalence of coexisting chronic kidney disease and hepatic cirrhosis is increasing due to the growing prevalence of both diseases.
2. A tight follow-up regimen is necessary in these patients in order to determine when to start dialysis based on the difficulty in determining the exact glomerular filtration rate and the presence of similar symptoms.
3. The spontaneous tendency towards arterial hypotension, haemodynamic instability, and increased risk of bleeding in cirrhotic patients make haemodialysis a difficult task.
4. Since periodic evacuation paracentesis is the treatment of choice for cirrhotic patients with ascites, the continuous and slow drainage of peritoneal liquid through peritoneal dialysis is similar to the normal treatment given to these patients, even on a long-term basis.
5. The fear of increased risk of peritonitis with peritoneal dialysis has not been clearly supported or rejected, and fewer bleeding complications and better haemodynamic tolerance have been observed, as well as decreased transmission of hepatitis B and C, when compared to haemodialysis.
6. As such, peritoneal dialysis should be considered as a viable and effective dialysis alternative for this group of patients.
Table 1. Child-Pugh classification of severity of liver disease
Table 2. Advantages and disadvantages of peritoneal dialysis in patients with chronic kidney disease and liver disease
Table 3. Summary of cirrhotic patients on peritoneal dialysis