El factor de crecimiento fibroblástico 23 (FGF-23) es una hormona derivada del hueso que participa en la regulación de la homeostasis del fósforo. Los niveles de FGF-23 se encuentran extremamente elevados en la enfermedad renal crónica y existe evidencia del papel de esta hormona en la patogénesis de los trastornos óseos y minerales en esta situación. Más aún, datos recientes implican al FGF-23 en la patogénesis de otras complicaciones sistémicas asociadas a las alteraciones óseo-minerales de la enfermedad renal crónica. La evidencia creciente de que las alteraciones del metabolismo mineral no se limitan a la enfermedad ósea ha acentuado el interés por la patofisiología y el tratamiento de las alteraciones del metabolismo mineral en la enfermedad renal crónica. Se ha propuesto que el aumento de FGF-23 es la respuesta inicial en los estadios precoces de la enfermedad renal crónica a la necesidad de proteger al organismo de los efectos adversos de la retención de fósforo. Estos aumentos de FGF-23 se asocian al riesgo creciente de mortalidad cardiovascular en los enfermos renales crónicos y son mediadores directos de toxicidad cardíaca. En esta revisión procuramos presentar aspectos relevantes de la fisiología del FGF-23 en la biología ósea y en la homeostasis mineral, así como en la fisiopatología de la enfermedad renal crónica y sus implicaciones clínicas.
Fibroblast Growth Factor 23 (FGF-23) is a bone-derived hormone involved in the regulation of phosphate homeostasis. FGF-23 levels are extremely elevated in Chronic Kidney Disease (CKD) and there is evidence supporting the role of this hormone in the pathogenesis of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Furthermore, recent data associates FGF-23 with the pathogenesis of systemic complications of CKD-MBD. The increasing evidence that the consequences of abnormal mineral metabolism are not restricted to bone disease changed the approach to the pathophysiology and treatment of disturbed bone and mineral metabolism in CKD patients. FGF-23 has been proposed to be the initial adaptive response in early CKD to protect the organism from the adverse effects of phosphate retention. Increased levels of FGF-23 observed in CKD patients are associated with cardiovascular mortality risk and was shown to mediate direct, "off-target" toxicity to the heart. This report aims to review the relevant aspects of the physiology of FGF-23 in bone biology and mineral homeostasis and the role of FGF-23 in the pathophysiology of CKD-BMD and its clinical implications.
INTRODUCTION
Chronic Kidney Disease (CKD) is a worldwide public health issue associated with several adverse outcomes, including cardiovascular disease and premature death.1 Although CKD encompasses a broad spectrum of different pathophysiologic processes, disorders of bone and mineral metabolism develop in virtually all patients as their kidney function declines.2 The discovery of Fibroblast Growth Factor 23 (FGF-23) happened in the sequence of the study of several rare hypophosphatemic disorders like X-linked Hypophosphatemia or Tumor Induced Osteomalacia.3 The elevated levels of FGF-23 observed in CKD patients4,5 were noticed very soon after the identification of this hormone. FGF-23 is a phosphaturic hormone increasing renal phosphate excretion and also reduces 1,25-dihydroxyvitamin D synthesis at the kidney level and increases its degradation. In early CKD, FGF-23 may be the first marker of disrupted mineral metabolism6 and the pivotal player in the pathophysiology and progression of Chronic Kidney Disease - Mineral and Bone Disorder (CKD-MBD), which is associated not only with bone abnormalities but also cardiovascular disease and vascular calcification.5 Recently, FGF-23 has been associated with several adverse clinical outcomes, such as the development of secondary hyperparathyroidism6 and left ventricular hypertrophy (LVH),7 as well as faster deterioration of renal function8 and increased mortality.9
CHRONIC KIDNEY DISEASE-MINERAL AND BONE DISORDER (CKD-MBD): A PARADIGM SHIFT
In the past, the disruption of mineral homeostasis in CKD was seen as the alteration of calcium and phosphorus levels and the dysregulation of the PTH and 1,25-dihydroxyvitamin D axis, which would eventually lead to secondary hyperparathyroidism10 and bone disease. Together, these abnormalities were known as "renal osteodystrophy".11 With the increasing evidence that the deleterious consequences of disturbed mineral metabolism in CKD were not restricted to the development of parathyroid and bone disease but were also associated to vascular calcification and cardiovascular morbidity and mortality,5,12 the Kidney Disease Improving Global Outcomes working group gathered in 2006 to rework the definition of renal osteodystrophy. Together, these abnormalities in calcium, phosphorus, PTH and vitamin D metabolism, bone turnover, mineralization, volume, strength and linear growth and cardiovascular or soft tissue calcification, all due to CKD, were re-named "Chronic Kidney Disease - Mineral and Bone Disorder", reflecting their systemic and complex nature.5 This new concept also fits well in terms of pathophysiology, since the mechanisms behind the various manifestations are closely entwined.11
OVERVIEW OF FGF-23
FGF-23 is a 32 kDa phosphate-regulating hormone secreted mainly by osteocytes and osteoblasts.13 This hormone inhibits renal phosphate reabsorption and reduces systemic levels of 1,25-dihydroxyvitamin D,14 leading to diminished phosphate load as a consequence of decrease renal tubular reabsorption and absorption from the gastrointestinal tract. FGF-23 performs these hypophosphatemic actions through the downregulation of type II sodium-phosphate cotransporters (NaPhT-2a and NaPhT-2c) localized in the apical membrane of the proximal convoluted tubule (PCT),14,15 inhibition of 25-hydroxyvitamin D 1α-hydroxylase and the stimulation of 25-hydroxyvitamin D-24-hydroxylase.14,16 Also FGF-23 is able to interact with PTH. FGF-23 directly suppresses PTH mRNA in vitro and decreases serum PTH in vivo.17 FGF-23 is elevated in several hereditary and acquired hypophosphatemic disorders, including X-linked hypophosphatemia, Autosomal Dominant Hypophosphatemic Rickets (ADHR) or Tumor Induced Osteomalacia (TIO). These disorders share a common phenotype of phosphaturia, hypophosphatemia and rickets/osteomalacia.18 In contrast, Hyperphosphatemic Familial Tumoral Calcinosis is characterized by soft tissue calcifications, hyperphosphatemia and elevated 1,25-dihydroxyvitamin D levels due to reduced levels of active FGF-23.19
FGF-23 has affinity to various receptors such as Fibroblast Growth Factor Receptor 1C 3C and 4. The regulation of phosphate reabsorption is mediated by its binding to the Fibroblast Growth Factor Receptor 1C (FGFR-1C) and to the co-receptor α-Klotho, which increases the binding affinity of FGF-23 for FGFRs. FGFR 3C and 4 seem to play a greater role in the regulation of 1,25-dihydroxyvitamin D metabolism.20
The co-receptor α-Klotho is a 130 kDa transmembrane protein expressed mainly in the distal convoluted tube (DCT), parathyroid and vascular tissue.21 Another report does not support Klotho-mediated FGF-23 effects in the vasculature22 but confirmative studies in humans are needed. Klotho was accidentally discovered as a mutated gene in mice with a premature aging disorder23 and its name comes from the Greek mythology: Klotho was the goddess who spun the thread of life. Conveniently, its anti-aging proprieties were afterward demonstrated in animal studies.24 Further investigation demonstrated that when α-Klotho is absent, the activity of FGF-23 is essentially suppressed.25 In addition to membrane-bound α-Klotho, the co-receptor also exists in two circulating forms, generated by different processes - alternate RNA splicing or proteolytic cleavage.26 Circulating α-Klotho is also capable of enabling FGF-23's signal transduction, but at a lower level than the membrane-bound form.18
FGF-23 diminishes α-Klotho expression,27 maybe through reductions in 1,25-dihydroxyvitamin D levels, which is known to stimulate α-Klotho expression.28 The proteolytic cleavage product of α-Klotho was implicated in the direct regulation of FGF-23.29 This form of Klotho is capable of inducing FGF-23 production in osteocytes in vivo, which suggest the presence of a new endocrine feedback loop in mineral metabolism.30 In CKD, FGF-23 is markedly elevated5 and α-Klotho is severely depressed.31 Several other actions of Klotho not related to FGF-23 have been described32 but those are outside of the scope of this review.
FGF-23 AND BONE BIOLOGY
There is increasing evidence linking bone biology and FGF-23. This hormone appears to coordinate renal phosphate handling to match bone mineralization and remodeling,13,33 which influences both the influx and efflux of calcium and phosphate from bone.34 Also, many studies suggest that FGF-23 may have a direct effect on bone with a direct inhibition of osteoblast differentiation and matrix mineralization in vitro,35 while a complete lack of FGF-23 results in impaired skeletal mineralization even with adequate phosphate, calcium and 1,25-dihydroxyvitamin D levels in mature animals.36 Consistent with these findings is the disruption of the Wnt-signaling pathway, which is responsible for osteoblast proliferation and bone matrix mineralization, noted in mice with excess FGF-23 expression in bone.37 In FGF-23 null mice the administration of a phosphate-deficient diet reversed hyperphosphatemia completely and greatly improved survival but the bone mineralization defects persisted.38 In a double FGF-23 and NaPhT-2a knock-out mouse model, the skeletal phenotype is similar to FGF-23 null mice, although the phosphate levels are not elevated.39 These studies not only suggest that the bone defects related to the absence of FGF-23 are independent of its actions on phosphate homeostasis, but also suggest that FGF-23 has a physiologic role in bone biology.
The finding of numerous single gene mutations leading to alterations in bone remodeling and increased FGF-23,37 also favor this hypothesis. Examples of these mutations are those affecting PHEX (phosphate-regulating gene with homologies to endoptidases on the X-chromossome)40 and members of the SIBLING protein family, such as DMP1 (dentrin matrix acidic phosphoprotein I)41 and MEPE (matrix extracellular phosphoglycoprotein).33 The exact mechanism by which these proteins regulate FGF-23 is yet to be determined. PHEX and DMP1 seems to exert its inhibitory effect through the FGFR1 receptor in the osteocyte.42 Increases in MEPE33 and loss of PHEX or DMP1 expression42 are both associated to defects in skeletal mineralization and increased levels of FGF-23. FGF-23 activity is also regulated at the protein level in which glycosylation by GALNT3 (GalNAc Transferase 3) protects it from the proteolytic cleavage by Furin,19 allowing the secretion of intact FGF-23 (iFGF-23), into circulation.43 iFGF-23 is biologically active, while the product of cleavage by Furin, the C-terminal form (cFGF-23) is not,3 but may be able to block the bioactivity of the intact form.44
Despite no detectable Klotho mRNA on bone,23 very recently the expression of FGF-23 and α-Klotho mRNA and protein were detected in porcine growth plate chondrocytes,45 which further highlights a potential role for FGF-23 in bone physiology. Shalhoub and colleagues demonstrated that supraphysiologic levels of FGF-23, in the presence of soluble α-Klotho, increase osteoblastic MC3T3.E1 cell proliferation and inhibit bone mineralization in vitro26 but, contrary to what was described by Wang et al.,35 over-expression of FGF-23 alone did not affect proliferation or mineralization.26 Samadfam et al., showed in mouse models that a sustained reduction in bone turnover increased FGF-23,46 which is concordant with its effect on bone mineralization. In addition, Wesseling-Perry and coleagues observed an inverse relationship between FGF-23 and osteoid thickness, osteoid maturation time and mineralization lag time in pediatric stage 5 CKD patients with secondary hyperparathyroidism, suggesting an association between FGF-23 and improved indices of skeletal mineralization in this population.47 Although these data seems contraditory it clearly shows the complexity of the mechanisms by which FGF-23 is associated with skeletal mineralization. Further research is required to clearly define FGF-23's role on bone biology and its possible implications in CKD-MBD.
SYSTEMIC REGULATION OF FGF-23: A COMPLEX SCENARIO
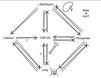
FGF-23 is regulated by phosphorus,48,49 1,25-dihydroxyvitamin D50 and probably also by PTH17 (Figure 1). A dietary phosphorus increase and 1,25-dihydroxyvitamin D augment FGF-23 levels, while dietary restriction of phosphorus has the opposite effect.48-50 The observation of the effect of phosphorus on FGF-23 is mostly derived from feeding studies in healthy humans and evidence of a direct effect is currently lacking. In fact, when serum phosphate was elevated through non-dietary sources, FGF-23 stayed unaltered,51 and in the feeding studies the changes in serum phosphate did not precede changes in FGF-23, as would be expected.48,49 This phosphate-sensing mechanism that regulates FGF-23 remains a mystery and might involve an unidentified phosphate sensor18 or be the indirect result of alterations in bone mineralization due to phosphate.52 The effects of 1,25-dihydroxyvitamin D on FGF-23 are thought to be controlled by a vitamin D response element (VDRE) in the FGF-23 promoter gene.53 Although this VDRE is present in both mouse53 and rat,54 and there is support for a gatekeeper effect,55 no analogous region has yet been found within the FGF-23 promoter of the human gene.18 The complexity of this system is also supported by the finding that in the absence of the Vitamin D Receptor, the increase in dietary phosphate does not increase FGF-23.56
Like 1,25-dihydroxyvitamin D, PTH seems to share a negative endocrine feedback loop with FGF-23. PTH stimulates the synthesis of FGF-23 both directly57 and indirectly via PTH-mediated increases in 1,25-dihydroxyvitamin D58 while PTH secretion is inhibited via a FGFR-Klotho-dependent pathway.17 However FGF-23 does not prevent the development of hyperparathyroidism (HPT) and there is a strong link between the FGF-23 increase and the severity of HPT in CKD,25 suggesting a possible causality effect.23 Furthermore, in the absence of PTH, FGF-23 levels are high rather than diminished,59 suggesting that PTH is not essential to increase FGF-23 secretion in humans. Also, in hypoparathyroidism FGF-23 action seems to be crippled, perhaps because of the absence of PTH or the low levels of 1,25-dihydroxyvitamin D.59 Indeed, inducing hypoparathyroidism with cinacalcet in patients with a FGF-23 excess disease like TIO attenuates FGF-23 action60 and cinacalcet seems to lower FGF-23 levels in CKD.61 This dynamic interplay between PTH and FGF-23 needs to be further clarified but it is already well established that both hormones are needed to maintain serum phosphate homeostasis.
A high calcium diet increased FGF-23 secretion both in wild type mice and in vitamin D receptor-ablated mice.56 This supports a stimulatory effect of calcium on FGF-23 secretion since other possible stimuli (PTH and 1,25-dihydroxyvitamin D) were decreased because of hypercalcemia or blunted in vitamin D receptor-ablated mice. In addition, studies show that non-calcium based phosphate binders like sevelamer, reduce FGF-23 more than calcium-based phosphate binders despite comparable reductions on serum phosphate,62 supporting the hypothesis of a direct effect of calcium on FGF-23. Finally, in the hypercalcemia of malignancy, FGF-23 levels are elevated despite PTH being suppressed.63 Hypocalcemia was associated with low FGF-23 in rats fed with a low calcium and low vitamin D diet,64 despite high serum levels of PTH and 1,25-dihydroxyvitamin D.65 This effect was reversed by the administration of calcium. Although we wait for further elucidation, this stimulatory effect of calcium on FGF-23 is undoubtedly important and should be taken into account in the therapeutic management of CKD-MBD in the years to come.
Finally, a potential role for iron in the regulation of FGF-23 is emerging. In the CKD population, especially in patients undergoing dialysis, parental iron therapy with erythropoietin is widely used to manage anemia. After some studies showing an association between iron therapy and bone mineral abnormalities, investigators began to speculate that intravenous iron administration could be responsible for an increase in FGF-23 levels and play a role in the development of hypophosphatemic osteomalacia during iron therapy.66,67 In fact, intravenous iron is capable of increasing FGF-23 secretion and induce hypophosphatemia in non-CKD patients.68 In stage 5 CKD population results are contradictory. Takeda et al., concluded that intravenous saccharated ferric oxide induces a small increase in the already elevated FGF-23 levels in hemodialysis patients69 while Deger et al., unexpectedly found a negative relationship between iron administration and serum iFGF-23 levels in a dialysis population.70 There is also contradictory data suggesting that iron deficiency increases FGF-23 expression in both wild-type mice and healthy individuals.71,72 In these populations, the serum iron levels negatively correlated with cFGF-23 but not with iFGF-23 while in ADHR subjects low serum iron was associated to an increase in both cFGF-23 and iFGF-23, which results in hypophosphatemia.71,72 Since ADHR is caused by an FGF-23 mutation that renders it resistant to cleavage,73 it can be postulated that iron deficiency does not increase iFGF-23 in healthy subjects because it is cleaved by Furin into smaller fragments which are then released and able to be detected with the C-terminal assay.74 Better understanding of the bone cell pathways related to this iron/FGF-23 interaction is required to determine the actual role of iron and the real importance of FGF-23 processing in the regulation of active FGF-23 levels.
PATHOPHYSIOLOGY OF CKD-MBD: IS IT FGF-23-CENTRIC?
There is still a lot of controversy regarding the pathophysiology of CKD-MBD. Actually there is no consensus on what induces/exacerbates CKD-MBD or the "right" order of appearance of the various factors involved. Several studies showed that FGF-23 levels are elevated in patients with various degrees of CKD and rise progressively as renal function declines4,75 (Figure 2).76 The exact CKD stage where FGF-23 levels start to increase is not completely clear and different studies have some inconsistent results.4,74 Also, elevated levels of FGF-23 were consistently associated with higher serum phosphate, higher fractional excretion of phosphate, lower estimated glomerular filtration rate (eGFR) and lower levels of 1,25-dihydroxyvitamin D.4,74 This latter association is independent of eGFR. It has been proposed that the rise of FGF-23 in early CKD is due to a compensatory response to intermittent increases in enteral phosphate burden because of the impaired renal excretion,10 diminished expression of α-Klotho that induces resistance to FGF-231 or due to alterations in osteocyte biology77,78 that somehow stimulates FGF-23 secretion directly.46 Others suggest that PTH levels increase first and result in elevated FGF-23 concentrations.17,48
The current data shows that FGF-23 expression in osteocytes is increased in early CKD, occurring as early as stage 2 at a time when 1,25-dihydroxyvitamin D, PTH, calcium and phosphate are still within normal range.79 DMP1 is also up-regulated at this stage,77 which appears to be an effort of the osteocyte to suppress FGF-23 production. In addition, serum FGF-23 levels are augmented in 30-40% of adults with an estimated GFR of 70-90ml/min/1.73m2 and serum phosphate was actually a bit lower than in subjects with higher eGFR.6 This reduction in serum phosphate combined with increased urinary fractional excretion of phosphate, suggest they are product of FGF-23 elevation.6 Since reduced α-Klotho expression is expected to cripple the excretion of phosphate,23 the lack of an increase in serum phosphate in early CKD also does not favor the FGF-23 resistance model of CKD-MBD. Also, PTH concentrations rose in only 10% of this same population.6
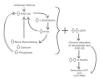
Although the initial trigger to the elevation of FGF-23 in early CKD is yet to be demonstrated, there is evidence to support that the early elevation in FGF-23 is the initial adaptive response in CKD80 and the first measurable biochemical evidence of disturbed mineral ion homeostasis in CKD-MBD.10 We can hypothesize (Figure 3) that this early FGF-23 elevation leads to a decrease in 1,25-dihydroxyvitamin D levels, as a consequence of inhibition of 25-hydroxyvitamin D 1α-hydroxylase rather than the loss of renal mass, as was traditionally thought.75 This decrease in 1,25-dihydroxyvitamin D levels is responsible for a secondary increase in PTH due to impaired intestinal calcium absorption (the transient hypocalcemia stimulates the calcium-sensing receptors in the parathyroid gland (PTG)) and loss of 1,25-dihydroxyvitamin D's negative feedback on the PTG. PTH would maintain normocalcemia at the expense of stimulating bone remodeling, the reduction of calcium excretion by the kidney and by increasing 25-hydroxyvitamin D 1α-hydroxylase activity. This augment in bone remodeling, not only increases bone calcium but also bone phosphate efflux, which has to be excreted by a declining number of functional nephrons. This increase in phosphate can further stimulate FGF-23 secretion. PTH is also capable of directly stimulating FGF-23 production.57 Furthermore, PTH-mediated effects on bone remodeling may lead to local alterations in bone biology that further amplify FGF-23 expression.52 This additional FGF-23 facilitates phosphate wasting by the kidney, but when renal function becomes severely affected, phosphate chronically stays above normal levels, further suppressing 25-hydroxyvitamin D 1α-hydroxylase activity and increasing PTH secretion (the elevation in intracellular phosphorus contributes to the increase in lifespan of PTH mRNAs11). In parallel, this state of chronic FGF-23 excess induces a down-regulation in α-Klotho expression, which clearly has a critical role in the progression of CKD-MBD.1 This reduced α-Klotho expression along with reduced FGFR expression in the PTG81 is responsible for the resistance to the inhibition of PTH expression by FGF-23 and contributes to the coexistence of secondary increases in both FGF-23 and PTH in CKD-MBD.82 In addition, the expression of the receptors for calcium83 and 1,25-dihydroxyvitamin D84 also decline, eventually leading to the development of an PTG secreting PTH autonomously of the suppressing stimuli.11 Thus, in the late stages of CKD, low levels of 1,25-dihydroxyvitamin D, hypocalcemia, hyperphosphatemia, reduced expression of α-Klotho and high levels of FGF-23 all contribute to the development of secondary hyperparathyroidism85 and CKD-MBD.
FGF-23 AND CLINICAL OUTCOMES
Besides the deleterious effects of the disruption of mineral metabolism, FGF-23 is an independent predictor of mortality,9 progression of renal disease,8 LVH7 and possibly vascular dysfunction86 in CKD patients. Faul et al. recently demonstrated supraphysiologic levels of FGF-23 similar to those seen in late CKD were able to induce left ventricular hypertrophy in an α-Klotho knockout mice.87 Although, we still await other studies to confirm this effect, this results support the hypothesis of α-Klotho-independent and direct "off-target" toxicity of FGF-23. It may also explain the consistent association between FGF-23 and LVH and establish a bridge between higher FGF-23 and greater mortality risk in the CKD population. In fact, components of the uremic cardiomyopathy,88 such as progressive ventricular failure, arrhythmia and sudden death (in which LVH is an important mechanism), are more common causes of death in advanced CKD than acute myocardial infarction.89 Even if there is no consistently robust association between FGF-23 and artery calcification,1 α-Klotho deficiency has been implicated in the pathophysiology of vascular calcification,90 which may be an indirect effect of chronically elevated FGF-23 levels in CKD.
CLINICAL IMPLICATIONS IN CKD-MBD
The hypothesis presented on the chapter Pathophysiology of CKD-MBD: is it FGF-23-centric? has obvious limitations. It does not explain why FGF-23 increases in the first place and how FGF-23 and PTH interact in the early stages of CKD. It does not take into account the possible effects of hypocalcemia or the role of iron on FGF-23 expression. Furthermore, it offers a "static" view of the bone mineral metabolism and presupposes that every patient with CKD progresses at the same speed. Yet, if it proves true, this integrated concept of the pathophysiology of CKD-MBD will have clinical implications. This hypothesis suggests that early CKD does not represent a true 1,25-dihydroxyvitamin D deficient state, but an adaptive response to protect against hyperphosphatemia, which means that FGF-23 may be a biomarker for earlier interventions in the CKD population. This could become an interesting tool to help interpreting low 1,25-dihydroxyvitamin D levels in the elderly population (e.g. a decrease due to FGF-23 in subclinical CKD versus true deficiency).80 Also, at advanced stages of CKD the main stimulus for FGF-23 elevation may be bone remodeling, supporting other interventional strategies targeting bone rather than the administration of 1,25-dihydroxyvitamin D.
Because 1,25-dihydroxyvitamin D further stimulates FGF-23 production, 1,25-dihydroxyvitamin D sparing therapeutic strategies could be important, such as combined low dose of active vitamin D compounds and calcimimetics, which are proven to lower FGF-23 in stage 5 CKD patients.91 Still, there is a paradox to be solved since epidemiological data has shown a survival advantage for CKD patients treated with active vitamin D analogs,92,93 and at the same time, vitamin D also increases FGF-23 and phosphate serum levels, contributing to calcifications,94 left ventricular hypertrophy7 and mortality.9 Wolf, M. proposed that the variability of FGF-23 response to active vitamin D therapy between populations may be the key to unravel this potential contradiction.1 Well designed, prospective, randomized, controlled clinical trials are clearly necessary comparing the effect of different therapeutic protocols in hard outcomes such as mortality.
CONCLUSION
In little more than a decade, the discovery of FGF-23 reforged our knowledge about mineral homeostasis in both health and disease. Still, we are just beginning to unravel the complex pathophysiologic process of CKD-MBD and many questions remain to be answered. What is the real role of FGF-23 in bone biology? What is the precipitating factor for the early FGF-23 increase in CKD? What is the role of iron? Does FGF-23 directly induce LVH? Is the current standard of care optimal? Our hope is that the resolution of these and other matters will help designing randomized clinical trials which will end in meaningful improvements in clinical outcomes for CKD patients in the future.
KEY CONCEPTS
- FGF-23 inhibits renal phosphate reabsorption and reduces the levels of 1,25-dihydroxyvitamin D.
- FGF-23 might have a physiologic role in bone biology and is complexly regulated both locally and systemically.
- FGF-23 is the initial adaptive response in early CKD and plays an important role in the pathogenesis of the systemic complications of CKD-MBD.
- The deleterious effects of the FGF-23 excess in CKD are due to both its physiologic actions and "off-target" toxicity.
Conflicts of interest
Receive Honoraria as speaker: João M. Frazão: Amgen, Sanofi, Abbott.
Figure 1. The complexity of the Kidney-Parathyroid-Bone Axis that regulates FGF-23.
Figure 2. Fibroblast Growth Factor 23 (1), 1,25-dihydroxyvitamin D (2), Parathyroid Hormone (3) and Phosphate (4) levels (y axis) according to Glomerular Filtration Rate (x axis).
Figure 3. Current hypothesis about the pathophysiology of Chronic Kidney Disease-Mineral and Bone Disorder.