Presentamos el caso de un paciente de 37 años que ingresa a cargo de Hematología, trasladado desde las Urgencias de Otorrinolaringología, donde había acudido por amigdalitis. Allí se demuestra anemia y leucopenia e ingresa con agranulocitosis en estudio. Un día más tarde el paciente presenta crisis blástica, y se le diagnostica de leucemia aguda con crisis mieloide. En dicha situación de crisis blástica el paciente inicia un cuadro de dolor lumbar brusco, con oliguria y deterioro de la función renal, seguido de anemización, en el contexto de un cuadro de hemólisis compatible con microangiopatía trombótica, por lo que somos consultados. Se inicia tratamiento con plasmaféresis y al día siguiente hemodiálisis (se realiza un total de 12 sesiones de plasmaféresis, hasta desaparecer los datos de hemólisis). Cinco días más tarde presenta cuadro de insuficiencia respiratoria, por el que pasa a la Unidad de Cuidados Intensivos, donde continúa con plasmaféresis y hemodiálisis. El paciente se mantiene en anuria desde entonces, con necesidad de hemodiálisis, sin ningún signo de recuperación renal. Una vez normalizadas las plaquetas, con tratamiento quimioterápico hematológico, se realiza biopsia renal percutánea, que confirma el diagnóstico de necrosis cortical. Finalmente el paciente queda incluido en programa sustitutivo de la función renal mediante hemodiálisis periódica.
A 37-year-old patient was transferred to Haematology from the ENT Emergency Department where he had been admitted due to tonsillitis. He displayed anaemia and leukopenia and had agranulocytosis in the study. A day later the patient had blast crisis, and was diagnosed with myeloid acute leukaemia. Due to blast crisis the patient experienced sudden back pain, with oliguria and renal function deterioration followed by anaemia, in the context of haemolysis consistent with thrombotic microangiopathy, and as such, we were consulted. We began treatment with plasmapheresis and on the following day we performed haemodialysis (we carried out a total of 12 sessions of plasmapheresis until haemolysis disappeared). Five days later there was respiratory failure, and the patient was consequently transferred to the Intensive Care Unit, where he continued treatment with plasmapheresis and haemodialysis. The patient remained anuric thereafter, requiring haemodialysis, with no sign of renal recovery. Once platelet levels normalised with haematology chemotherapy, a percutaneous renal biopsy was performed, which confirmed the diagnosis of cortical necrosis. Finally, the patient underwent renal replacement therapy by regular haemodialysis.
Case study
Our patient is a 37-year-old male without any relevant medical history, except for recurrent tonsillitis, being an ex-smoker and occasional cannabis user. He came to the Emergency Department due to severe odynophagia lasting 24 hours. Upon physical examination, there was nothing remarkable, except that the patient was slightly overweight (body mass index: 29kg/m2), with hyperaemic tonsils. Given the patient’s poor general condition, low-grade fever (37.3ºC) and tonsils that inhibited swallowing, it was decided to admit the patient to the ENT Department on 25 April 2013.
The day after admission, the patient was transferred to Haematology due to agranulocytosis in the study, after the test showed mild anaemia (haemoglobin [Hb] 11.9g/dl), leukopenia (340/l), platelets 270,000/l, with plasma creatinine (PCr) of 0.9mg/dl, normal coagulation, with an extension in peripheral blood with neutropenia, without dysmorphias or immature cells, with borderline macrocytosis and normal platelets. Throat cultures were taken along with cytomegalovirus, Epstein-Barr, rubella, toxoplasmosis, human immunodeficiency virus and herpes serologies, which were negative.
The day after the transfer, a control test showed 8290 leukocytes, Hb of 11g/dl, 20,000/l platelets, urea of 59mg/dl and Pcr of 2.3mg/dl. A peripheral blood smear was taken again, in which a pathological population of immature cells was detected with a myeloid appearance and intense granulation. Some of them had a 2-lobed cleft nucleus and erythroblastosis with atypical promyelocytes. This was consistent with acute myeloblastic leukaemia that was probably promyelocytic and a myelogram was subsequently performed, which confirmed promyelocytic cell infiltration. A cytogenetic study was carried out, which confirmed translocation (t15;17). On the same day, we were consulted because the patient had sudden abdominal pain at the lumbar level during the night, with oligoanuria. We reviewed the peripheral blood smear, in which we detected significant schistocytosis at around 8%. In this context, the diagnosis of thrombotic microangiopathy was likely. On 27 April 2013 we performed a computerised tomography (CT) scan of the abdomen, which revealed a perfusion defect bilaterally at the renal cortex level with permeable renal arteries and veins, consistent with cortical necrosis. We also performed a renal scintigraphy, which was indicative of cortical necrosis. In the test carried out 6 hours later, the patient already displayed sediment with microscopic haematuria and proteinuria of 100mg/dl; the blood test displayed Pcr of 4.6mg/dl, with a slight elevation in liver enzymes, with ultra-sensitive C-reactive protein (CRP) of 165mg/l, total bilirubin of 5.9mg/dl, with coagulation (D-dimer of 36mg/l, prothrombin activity of 53%, with the rest being normal), normal direct and indirect Coombs tests, and therefore, given the high clinical suspicion of haemolytic-uraemic syndrome (HUS), we started treatment with daily plasmapheresis with 5 litres of fresh frozen plasma (1.5 plasma volumes), with hypotensive therapy, given the progressive increase in blood pressure: 120/70 to 170/90mmHg. The next day, the patient remained oliguric with Pcr of 7mg/dl and high blood pressure (160/80mmHg), and therefore we began haemodialysis (first session: 28 April 2013). We carried out a total of 12 plasmapheresis sessions (7 consecutive and 5 on alternate days), until haemolysis disappeared (a decrease in lactate dehydrogenase [LDH], indirect bilirubin and schistocytes [Figure 1], along with an increase in haptoglobin: mg/dl [70 (1 May 2013 ) to 75 ( 13 May 2013 ) to 217 (17 June 2013 )], and platelets). On 1 May 2013, after the fifth plasmapheresis session, the patient had severe respiratory failure with mild haemoptysis and decreased consciousness, and as such he was transferred to the intensive care unit (ICU), where he continued with plasmapheresis and haemodialysis. In the ICU, mechanical ventilation was required and a lung scintigraphy was carried out due to suspected pulmonary infarction with no signs of pulmonary thromboembolism; a CT scan of the head was also performed. Finally, the patient left the ICU four days later spontaneously breathing with a respiratory infection due to Acinetobacter and Pseudomona aeruginosa and a urinary infection due to Acinetobacter baumanii. The clinical response to chemotherapy with ATRA and idarubicin was satisfactory and the patient showed a good response to treatment. He was discharged by haematology on 18 June 2013 with revisions in outpatient clinics.
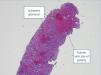
From the renal point of view, the patient remained anuric thereafter and required haemodialysis, without any sign of recovery. We carried out a control CT angiogram, which displayed cortical hypoperfusion. Once platelet levels normalised with chemotherapy, we performed a percutaneous renal biopsy (7 June 2013): 19 glomeruli, 16 of which displayed severe changes typical of coagulative necrosis with complete destruction of all cellular elements secondary to ischaemia. In three of them, we observed the same signs, but with preservation of their nuclei, as well as mesangiolysis, thickening of the membranes with the presence of red blood cells (schistocytes), and occasional neutrophils (PMN) without double contours or crescents or fibrinoid necrosis the glomerular capillary or the afferent arteriole. In the interstitial area, there was diffuse cortical necrosis with the presence, in the cortico-medullary and medullary junction, of tubules with red blood cell casts, haemosiderin pigment, cellular ischaemic changes with brush border loss and marked nuclear atypia and chronic inflammatory infiltrate and mild fibrosis patches. At vascular level, there were arteries with a medium-sized diameter, the presence of intimal mucoid degeneration with luminal occlusion and incipient myointimal fibrous changes, which confirmed the diagnosis of coagulative cortical necrosis with vascular changes consistent with HUS (Figures 2 and 3) and as such, given the low expectation of renal recovery, we requested an assessment by Vascular Surgery for a vascular access, with the patient being included in a renal replacement programme through regular haemodialysis.
Diagnosis
Renal failure due to bilateral renal cortical necrosis caused by ischaemia due to thrombotic microangiopathy in the context of acute myeloid leukaemia with myeloid crisis.
DISCUSSION
Cortical necrosis accounts for less than 2% of all causes of acute renal failure in adults and represents 15%-20% of acute renal failure in the third trimester of pregnancy. Renal cortical necrosis may have several degrees of extension: minimal, focal and massive and may be unilateral or bilateral. Our patient’s necrosis was bilateral and massive, given his poor clinical progression. Generally, the subcapsular and juxtamedullary cortical tissue is preserved, except in cortical necrosis due to rejection, in which the whole cortex tissue can become necrotic.1,2
Its most common causes include obstetric complications (50%-70%), and non-obstetric causes represent the remaining 20%-30%. Premature placental abruption is the most common obstetric cause. HUS is the most common non-obstetric cause (around 50%-70% of the cases), followed by septic situations. Other causes are severe dehydration, acute and hyperacute kidney rejection, biological venoms and ethylene glycol, etc.
The exact mechanism behind renal cortical necrosis is unknown. It is caused by a significant decrease in renal arterial perfusion secondary to vascular spasm, or microvascular injury or intravascular coagulation, and it is the pathological progression of acute tubular necrosis that results in cortical necrosis. Clinically, it usually presents with anuria, and requires haemodialysis on most occasions. Among the diagnostic tests, imaging tests play a key role in preventing the risk of bleeding involved in renal biopsies in these patients, due to their common clotting disorders. The technique normally used to carry out the diagnosis is angiography, which in addition to being a diagnostic tool is also useful for the prognosis of renal function, since better renal perfusion results in a better prognosis of recovery. However, CT angiography or renal scintigraphy are also valid techniques that are useful for diagnosis.3 Its prognosis is poor, with a mortality rate of around 50%. The calcium deposits observed in the renal cortex suggest renal cortical necrosis, but the latter appear late in the course of the disease as a result of chronicity and are only found in 20%-50% of cases.4-7
In the prodromal phase of atypical HUS upper respiratory infection (23%), unclassified fever (46%) as our patient displayed, and vomiting (81%) have been observed. The histological lesion characteristic of atypical HUS is arterial thrombotic microangiopathy, while in the typical HUS group the most common lesion was glomerular thrombotic microangiopathy, which corresponds to that observed in our patient. Renal cortical necrosis is the most serious complication in this profile and most patients require dialysis.8,9
In summary, we must say that, with regard to the microangiopathy that the patient displayed, in the context of the blast crisis, HUS was secondary to a tumour process, and that the aforementioned explosion of atypical cells resulted in the acute situation, which led to renal failure due to renal cortical necrosis.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Figure 1. Graph showing progression.
Figure 2. Renal cast.
Figure 3. Image of a typical artery in secondary haemolitic uraemic syndrome.