Assessment of volume status and differentiating “underfill” and “overfill” edema is essential in the management of patients with nephrotic syndrome (NS).
ObjectivesOur aim was to evaluate the volume status of NS patients by using different methods and to investigate the utility of bioelectrical impedance analysis (BIA) in children with NS.
MethodsThe hydration status of 19 patients with NS (before treatment of NS and at remission) and 25 healthy controls was assessed by multifrequency BIA, serum N-terminal-pro-brain natriuretic peptide (NT-proBNP) levels, inferior vena cava (IVC) diameter, left atrium diameter (LAD) and vasoactive hormones.
ResultsRenin, aldosterone levels, IVC diameter and LAD were not statistically different between the groups. NT-proBNP values were statistically higher in the attack period compared to remission and the control group (p=0.005 for each). Total body water (TBW), overhydration (OH) and extracellular water (ECW) estimated by the BIA measurement in the attack group was significantly higher than that of the remission group and controls. There were no significant correlations among volume indicators in group I and group II. However, significant correlations were observed between NT-proBNP and TBW/BSA (p=0.008), ECW/BSA (p=0.003) and ECW/ICW (p=0.023) in the healthy group. TBW was found to be higher in patients with NS in association with increased ECW but without any change in ICW. NT-proBNP values were higher in patients during acute attack than during remission.
ConclusionsOur findings support the lack of hypovolaemia in NS during acute attack. In addition, BIA is an easy-to-perform method for use in routine clinical practice to determine hydration status in patients with NS.
La evaluación del estado volumétrico y la diferenciación entre edema «por sobrellenado» y «por infrallenado» es fundamental en el manejo de los pacientes con síndrome nefrótico (SN).
ObjetivosNuestro objetivo fue evaluar el estado volumétrico de los pacientes con SN mediante el uso de diversos métodos y estudiar la utilidad del análisis de impedancia bioeléctrica (BIA) en niños con SN.
MétodosSe evaluó el estado de hidratación de 19 pacientes con SN (antes del tratamiento y en la remisión) y de 25 controles sanos mediante BIA multifrecuencia, valores plasmáticos de la fracción N-terminal del péptido natriurético cerebral (NT-proBNP), diámetro de la vena cava inferior, diámetro de la aurícula izquierda y hormonas vasoactivas.
ResultadosLa renina, los niveles de aldosterona, el diámetro de la vena cava inferior y el de la aurícula izquierda no fueron estadísticamente diferentes entre los grupos. Los valores de la NT-proBNP fueron estadísticamente más altos en el período de crisis que en el momento de remisión y que en el grupo de control (p=0,005 en cada uno). El agua total corporal (TBW), la hiperhidratación y el agua extracelular (ECW) estimada mediante la medición del BIA en el grupo de crisis fue considerablemente mayor que la del grupo de remisión y los controles. No hubo correlaciones importantes entre los indicadores de volumen en el grupo i y en el grupo ii. Sin embargo, se observaron correlaciones considerables entre NT-proBNP y TBW/BSA (p=0,008), ECW/BSA (p=0,003) y ECW/ICW (p=0,023) en el grupo sano. Se encontró que TBW fue mayor en los pacientes con SN relacionado con el aumento de ECW, pero sin ningún cambio en ICW. Los valores de la NT-proBNP fueron más altos en los pacientes durante la crisis aguda que durante la remisión.
ConclusionesNuestros hallazgos apoyan la falta de hipovolemia en el SN durante la crisis aguda. Además, BIA es un método fácil de utilizar en la práctica clínica habitual para determinar el estado de hidratación en pacientes con SN.
Edema is one of the principal symptom of nephrotic syndrome (NS) and remains one of the main reason for admission to the hospital. There have been two theories explaining the development of edema in nephrotic syndrome (NS), namely the under- and overfill hypotheses.1,2 The pathogenetic mechanism of edema in the NS was traditionally considered to be the result of a series of events, postulates that a decrement in oncotic pressure leads to excess filtration of fluid into the interstitial compartment causing hypovolemia, secondary hyperaldosteronism and secondary renal sodium retention. However, this hypovolemia concept or underfill hypothesis, cannot explain all features of volume regulation and sodium reabsorption in the nephrotic syndrome.3–5 Children with edema associated with idiopathic NS (INS) do not commonly have intravascular volume depletion; actually several studies suggest that children with INS may have normal or even increased intravascular pressure.6,7 The overfill hypothesis now suggests that edema is a result of a primary renal defect in sodium excretion that is independent of low oncotic pressure and is responsible for primary extracellular volume expansion, which leads to secondary leakage of fluid toward the interstitium.8,9
While the mechanism of edema is a theoretical topic of discussion, understanding the intravascular volume status of the patients accurately is a crucial issue while planning the treatment of edema. However, a significant issue in the consideration of this problem is the lack of a gold standard for the assessment of intravascular volume.10 Several noninvasive techniques for the assessment of hydration status exist. Monitoring of relative blood volume changes, inferior vena cava diameter (IVCD) measurements and biochemical markers such as atrial atriuretic peptide (ANP), brain natriuretic peptide (BNP) are indirect methods, which provide information about the intravascular filling. Although dilution methods are accepted as the most appropriate standards for fluid volume determination, they cannot be applied in routine clinical practice due to the patient availability for many hours and time-consuming assays of blood samples.10–12 In recent years, Bioelectrical impedance analysis (BIA) has found a place in the literature as a practical method for measuring body fluids particularly for patients on hemodialysis due to its advantages such as ease of application, non-invasiveness, and reproducibility.12–17
The present study aimed to determine the volume status using multifrequency BIA, NT-proBNP levels, IVC, LAD measurements to examine the relationship between various volume indicators and to investigate the utility of BIA in evaluating the volume status in children with INS.
Materials and methodsNineteen children (11 males, 8 females) with NS at debut or relapse admitted to our nephrology unit were prospectively studied from September 2012 to October 2013. The age range was 1–11 years (mean±SD, 5.13±2.53 years). Nephrotic syndrome was defined as severe proteinuria over 40mg/m2/h, hypoalbuminemia (<2.5g/dL), and edema. Relapse was defined as three consecutive days with plus two or more on urinary albumin dipstick. Remission was achieved when urinary dipstick was negative for protein for three consecutive days. Patients were classified as steroid resistant when remission was not achieved with prednisone therapy at 60mg/m2/day for 4 weeks. All patients were treated with prednisone, per International Study of Kidney Disease in Children regimen18 (started after routine workup for new NS patients and immediately upon admission in relapsed NS patients). Patients were excluded from the study if they met any of the following criteria: established cardiovascular disease, evidence of secondary NS and specific glomerulonephritis, usage of steroids or immunosuppressive drugs during the previous six months.
Twenty-five age and sex matched healthy volunteers served as the control group. These subjects had normal blood pressure (BP), no clinical electrocardiographic (ECG) or echocardiographic evidence of cardiovascular disease, and no evidence of pulmonary disease.
Study designA complete review was carried out for each patient which included the patient's past and family history, physical examination and data such as gender, age, height, weight, blood pressure (BP) on admission. Their clinical edema findings were appraised by the same pediatric nephrologist. Hypertension was defined as blood pressure of more than the 95th percentile for gender, age and height.19 Tachycardia was defined as a heart rate above the upper heart rate limit adjusted for age.
Biochemistry analyses, serum NT-proBNP, anthropometric and BP measurements, BIA, and IVC, left atrial diameter by echocardiography were performed at baseline in all participants. Blood and urine samples were collected for the laboratory assessments listed in the study protocol. Then, the serum samples were stored at −20°C for NT-proBNP measurements. Prednisolone treatment was initiated after the first evaluation of the patients. Physical examination, laboratory assessments, BIA, echocardiographic evaluation and NT- proBNP measurements were repeated during the remission period in the patients group.
Control data were used to compare the results of laboratory assessments, BIA, cardiovascular (IVC and left atrial diameter) and NT-proBNP measurements. This study was approved by the ethics committee of the Medical Faculty of Ondokuz Mayis University. Informed assent and consent were obtained from their parents before the study began.
According to the study group, the patients were evaluated into two categories: Debut or relapse of NS in admission (group I) and in remission after treatment (group II). Healthy volunteers served as the control group (group III).
Blood and urine samplesBlood and urine samples were collected at debut or relapse of NS and when stable remission (10–14 days after initiation of treatment) was achieved. The medication (steroids) was continued in unchanged dosages during the 10–14 days of study. Blood samples for measurements of serum creatinine, albumin, sodium and urine analysis of creatinine, sodium, protein excretion from spot urine samples were analyzed at the Department of Clinical Biochemistry using standard procedures. Fractional excretion of sodium (FENa, %) was calculated by on spot urine tests by the formula; serum aldosterone levels and plasma renin activity were determined by using Beckman Coulter RIA (Radioimmunoassay) kits with a Gamma counter device (ISO Data Multiwell Gamma Counter).
For determination of NT-proBNP, venous blood samples were collected in biochemistry tubes without anticoagulant and allowed to coagulate. The samples were centrifuged (4000rpm for 5min at +4°C) and the serum frozen at −20°C until analysis. The kits and serum samples were allowed to reach room temperature (+25°C) before the measurement. Serum NT-proBNP levels were measured by ELFA (enzyme-linked fluorescent assay) method using a VIDAS PC device (bioMERIEUX, France) with commercial NT-proBNP kits (VIDAS&NT-proBNP, bioMERIEUX, France) at the Investigational Laboratory of Ondokuz Mayis University School of Medicine. The results were expressed as pg/mL. The analytical measurement reference range of the NT-proBNP kit was 20–25,000pg/mL. The measurement was performed as per the procedure of the manufacturer.
Bioimpedance measurementTwo bioimpedance analysis (BIA) are currently available for use in clinical practice: single-frequency bioimpedance analysis and multifrequency bioimpedance spectroscopy (BIS). Single-frequency BIA, measures whole body impedance using one electrical current with a frequency of 50kHz. Therefore this cannot differentiate between extracellular and total body fluid resistances because this frequency of electrical currents do not pass cell membranes. The multifrequency bioimpedance spectroscopy (BIS) depends on a different electrical model. ECW and TBW resistances are determined using multiple imperceptible currents of varying frequencies and these water volumes are calculated from the respective impedances.20
We applied the Body Composition Monitor (BCM, Fresenius Medical Care) using multi-frequency BIS in order to assess the hydration status. Four electrodes were placed on each side on the dorsal surfaces of hands and feet of the supine patients. Two electrodes were dorsally placed in the metacarpophalangeal articulations and in the carpus of the hand respectively. The pair on the foot was located in the metatarsophalangeal and in the ankle articulation. Patients were connected to the device with these electrodes and measurements completed in 1–4min after entering gender, height (in cm), body weight (in kilograms), and blood pressure (systolic and diastolic mm Hg) data for each patient. Body composition analysis was performed by using Fluid Management Tool version 3.2.11 software. In cases with inadequate data quality, the measurements were repeated by changing the electrodes.
Results of measurements included that of overhydration (OH), total body water (TBW), extracellular water (ECW), intracellular water (ICW), body mass index (BMI). Bioimpedance spectroscopy (BIS) uses physiological modeling and mixture equations (Cole–Cole plot and Hanai formulae) to first determine the electrical resistance of ECW and ICW and then calculate the volumes of these respective compartments. This is essential for identification of OH. The BCM uses the BIS technique.20
The BCM states the body weight in means of lean tissue mass (LTM – mainly muscle), adipose tissue mass (ATM – mainly fat) and overhydration (OH). OH is almost 100% ECW, whereas the water of LTM and ATM consist differing ratio of ECW and ICW in addition to solid components. As the extracellular hydration of LTM and ATM is known, the estimated “normal” volume of ECW of these tissues can be calculated. The difference between “normal” ECW and measured ECW is the excess fluid, OH. Reference ranges are available for OH, lean tissue index (LTI), fat tissue index (FTI) and extracellular/intracellular (E/I) ratio.21 These ranges simplify identification of abnormal conditions by evaluating the patient's results to the reference population.
Echocardiographic measurementsEchocardiographic evaluations were performed at the time of BIA investigation by the same pediatric cardiologist, using the “Toshibo Aplio 770s Echocardiography System” with 3.5 and 5.5MHz probes as appropriate for age after resting (for 10–30minutes) in the supine position.
All examinations were measured IVCD was measured from subxiphoidal long axis position in 2cm to its junction to right atrium before the p wave in the electrocardiogram. The maximum diameter in expiration and the minimum diameter in deep inspiration were measured and indexed for body surface area (BSA). Left atrium diameter (LAD) was measured at the parasternal position. LAD was determined as diameter of left atrium (mm)/body surface area (m2). The patients were scanned during debut or relaps NS and at remission periods.
Statistical evaluationStatistical analyses were performed using “SPSS for Windows© 15.0” (Statistical Program in Social Sciences) package software. Data are presented as mean and standard deviation (SD) or as median and ranges (minimum–maximum) according to their distribution.
For the comparisons between the patient group and the control group, the independent samples t-test was used for the parameters with normal distribution and the Mann–Whitney U-test was used for the parameters with non-normal distribution. The pre- and post-treatment data of the patient group were compared using the paired samples t-test or the Wilcoxon rank-sum test. To determine the significance and strength of associations, we used the Pearson's correlation coefficient r for analyses of associations between continuous variables and Spearman rank for non-parametric variables. The p values below 0.05 were considered as statistically significant.
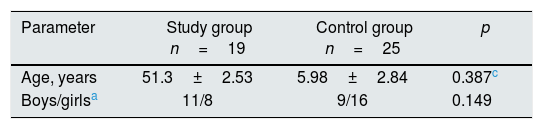
ResultsBaseline demographic and clinical and biochemical characteristics of the study populationA total of 19 patients with debut (n=14) or relapse (n=5) of NS and 25 healthy children were enrolled in the study. Demographic and clinical data of the study groups are reported in Table 1. In the present study, 17 patients were diagnosed with INS based on the clinical and laboratory findings, and steroid therapy was initiated without obtaining kidney biopsy. The diagnosis of MCNS was supported after biopsy in one patient who remained refractory to steroid therapy. Two patients older than 10 years were biopsied and then steroid treatment was started with the diagnosis of MCNS. 3 patients were excluded from group II because of refractory to steroid therapy.
Demographic, clinical and biochemical data of the of the study population.
| Parameter | Study group n=19 | Control group n=25 | p |
|---|---|---|---|
| Age, years | 51.3±2.53 | 5.98±2.84 | 0.387c |
| Boys/girlsa | 11/8 | 9/16 | 0.149 |
| Group I (debut/relaps) | Group II (remission) | Group III (control) | p (groups I–III) | p (groups I–II) | |
|---|---|---|---|---|---|
| BSAb (m2) | 0.71 (0.49–1.38) | 0.67 (0.49–1.08) | 0.76 (0.5–1.35) | 0.582d | 0.404e |
| BMIb (kg/m2) | 18.3 (15.6–22.7) | 17.9 (14.5–21.1) | 16.7 (13.7–24.2) | 0.01d | 0.327e |
| Systolic blood pressure, mmHg | 107.7±9.3 | 107.2±9.7 | 100.7±11.3 | 0.030c | 0.773f |
| Diastolic blood pressureb, mmHg | 70 (45–90) | 68 (40–90) | 60 (46–79) | 0.033d | 0.380e |
| P-albuminb (mg/dL) | 1.73 (1.1–2.6) | 4.39 (3.4–4.62) | 4.5 (4.5–4.99) | <0.001d | 0.001e |
| Proteinuriab (spot urine, mg/mg) | 11.5 (2.03–28.2) | 0.13 (0.10–0.68) | 0.16 (0.09–0.54) | <0.001d | 0.001e |
| FeNa+b (%) | 0.1 (0–0.67) | 0.47 (0.08–1.90) | 0.31 (0.05–11.2) | 0.005d | 0.002e |
| Reninb (ng/mL/h) | 2.76 (0.03–23.6) | 4.32 (0.38–13.96) | 1.98 (0.34–19.98) | 0.671d | 0.594e |
| Aldosteroneb (pg/mL) | 5.35 (0.96–82) | 27.27 (1.5–46.88) | 15.6 (0.77–210) | 0.151d | 0.225e |
Data are presented as means±standard deviations (x±SD) or as median with range.
The mean (±SD) age of the patient and control populations were similar (5. 1±2.53 vs. 5.98±2.84 years, respectively) and according to gender distribution, there were 8 girls and 11 boys in NS group and 16 girls and 9 boys in control group. There were no significant differences in age, gender, weight, height or BSA between the patient and control groups. The median BMI was significantly higher in group I than in group III [18.3 (15.6–22.7) vs. 16.7 (13.7–24.2) kg/m2; p=0.01]. There was no difference in the BMI values between group I and group II (data was shown in Table 1). The remaining comparison of the patients with NS and control group are given in Table 1. None of the patients had clinical signs of hypovolemia, such as tachycardia, pallor or abdominal pain. Eight (42.1%) were hypertensive at the time of investigation. Compared with healthy controls, group I had significantly higher systolic and diastolic blood pressure but not significant in the group I and the group II (Table 1) In addition to the significantly higher proteinuria and lower serum albumin concentrations, patients had lower FENa levels, the lowest in the group I. Table 1 shows the laboratory measurements, including serum albumin, spot urine/creatinine, FENa serum renin and aldosterone levels in patients and controls. All the laboratory measurements were statistically significant different in group I than in group III; however, there was no difference in serum renin and aldosterone levels among the groups (p>0.05).
Comparison between volume status assessment methodsPatients’ and healthy controls volume status were investigated at the same time using echocardiographically derived parameters (IVC), cardiac biomarkers (NT-proBNP) and BIA parameters.
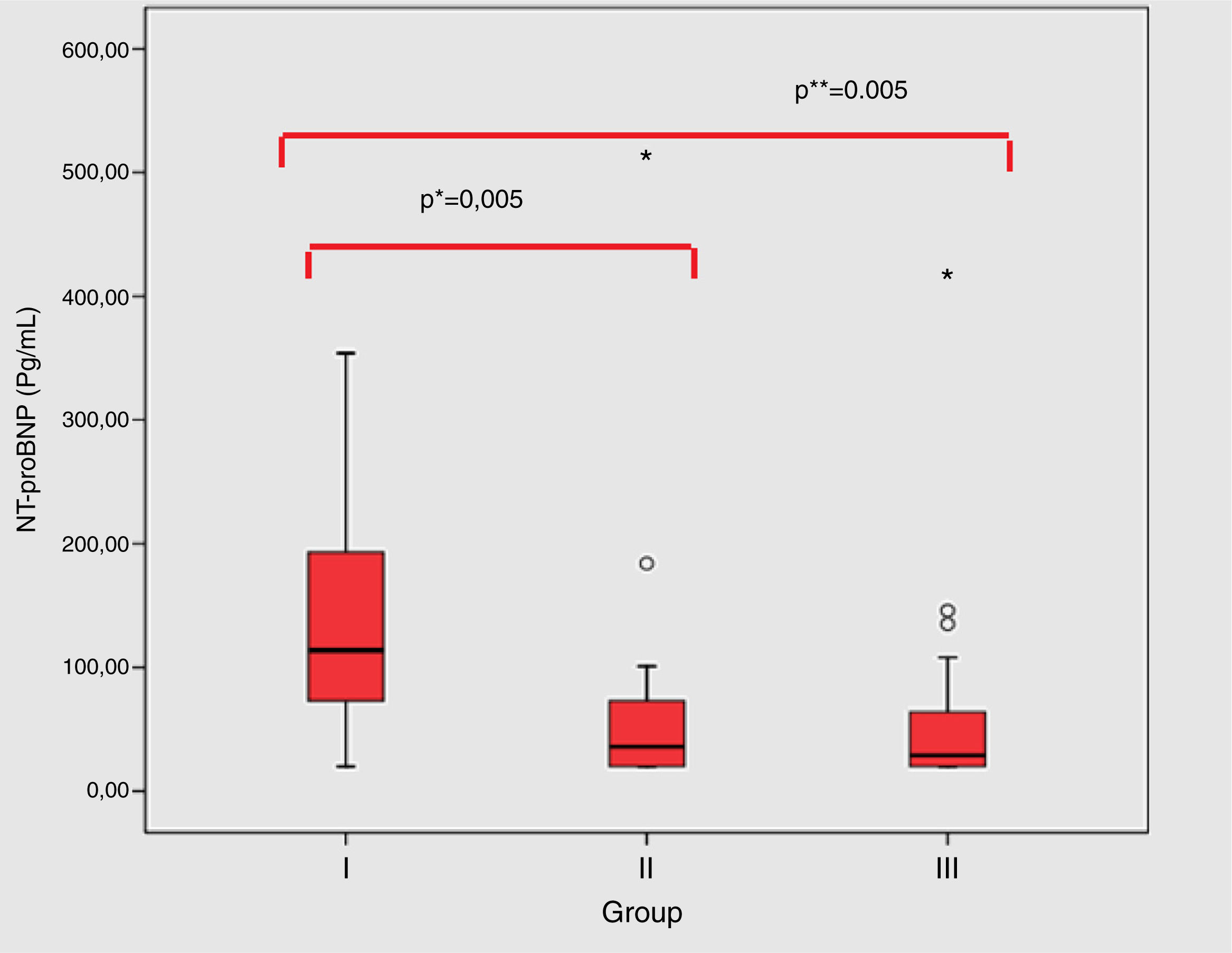
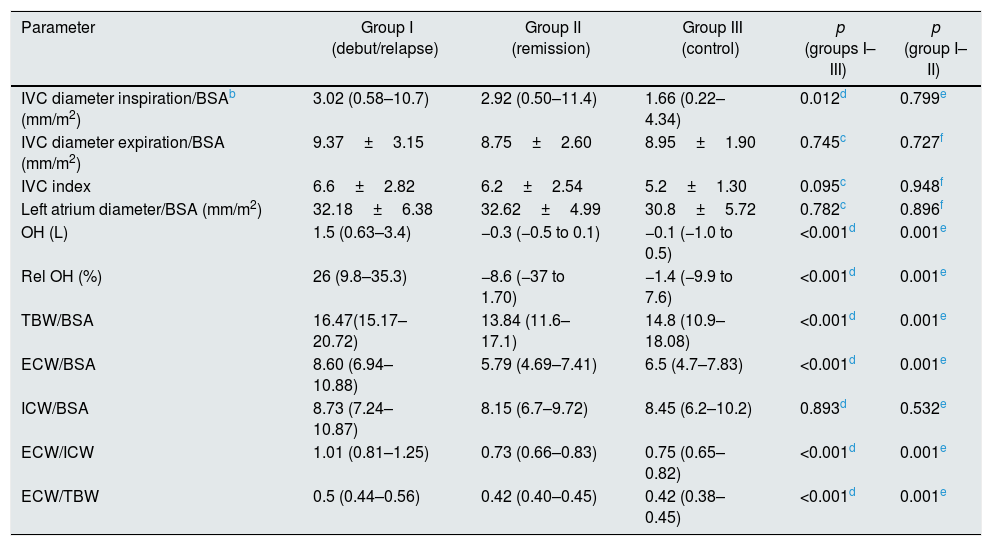
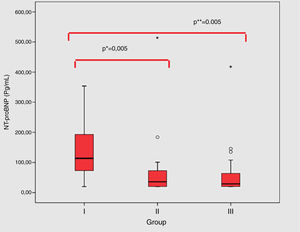
Group I patients’ had significantly higher NT-proBNP value in comparison to the group II (114 (20–1031) vs. 30 (20–514) pg/mL; p=0.005) and group III (114 (20–1031) vs. 29 (20–418) pg/mL; p=0.005) (Fig. 1). Table 2 shows the echocardiography indices indexed to BSA, including IVC diameter in inspiration, expiration and left atrial diameter. The median IVC diameter on inspiration/BSA was higher in group I (3.02 (0.58–10.7) mm/m2) than in group III (1.66 (0.22–4.34) mm/m2; p=0.012). No significant change in IVC diameter expiration/BSA, IVC index, left atrial diameter/BSA was found among the groups.
Assessment of ECW And Fluid Status in Study Population.
| Parameter | Group I (debut/relapse) | Group II (remission) | Group III (control) | p (groups I–III) | p (group I–II) |
|---|---|---|---|---|---|
| IVC diameter inspiration/BSAb (mm/m2) | 3.02 (0.58–10.7) | 2.92 (0.50–11.4) | 1.66 (0.22–4.34) | 0.012d | 0.799e |
| IVC diameter expiration/BSA (mm/m2) | 9.37±3.15 | 8.75±2.60 | 8.95±1.90 | 0.745c | 0.727f |
| IVC index | 6.6±2.82 | 6.2±2.54 | 5.2±1.30 | 0.095c | 0.948f |
| Left atrium diameter/BSA (mm/m2) | 32.18±6.38 | 32.62±4.99 | 30.8±5.72 | 0.782c | 0.896f |
| OH (L) | 1.5 (0.63–3.4) | −0.3 (−0.5 to 0.1) | −0.1 (−1.0 to 0.5) | <0.001d | 0.001e |
| Rel OH (%) | 26 (9.8–35.3) | −8.6 (−37 to 1.70) | −1.4 (−9.9 to 7.6) | <0.001d | 0.001e |
| TBW/BSA | 16.47(15.17–20.72) | 13.84 (11.6–17.1) | 14.8 (10.9–18.08) | <0.001d | 0.001e |
| ECW/BSA | 8.60 (6.94–10.88) | 5.79 (4.69–7.41) | 6.5 (4.7–7.83) | <0.001d | 0.001e |
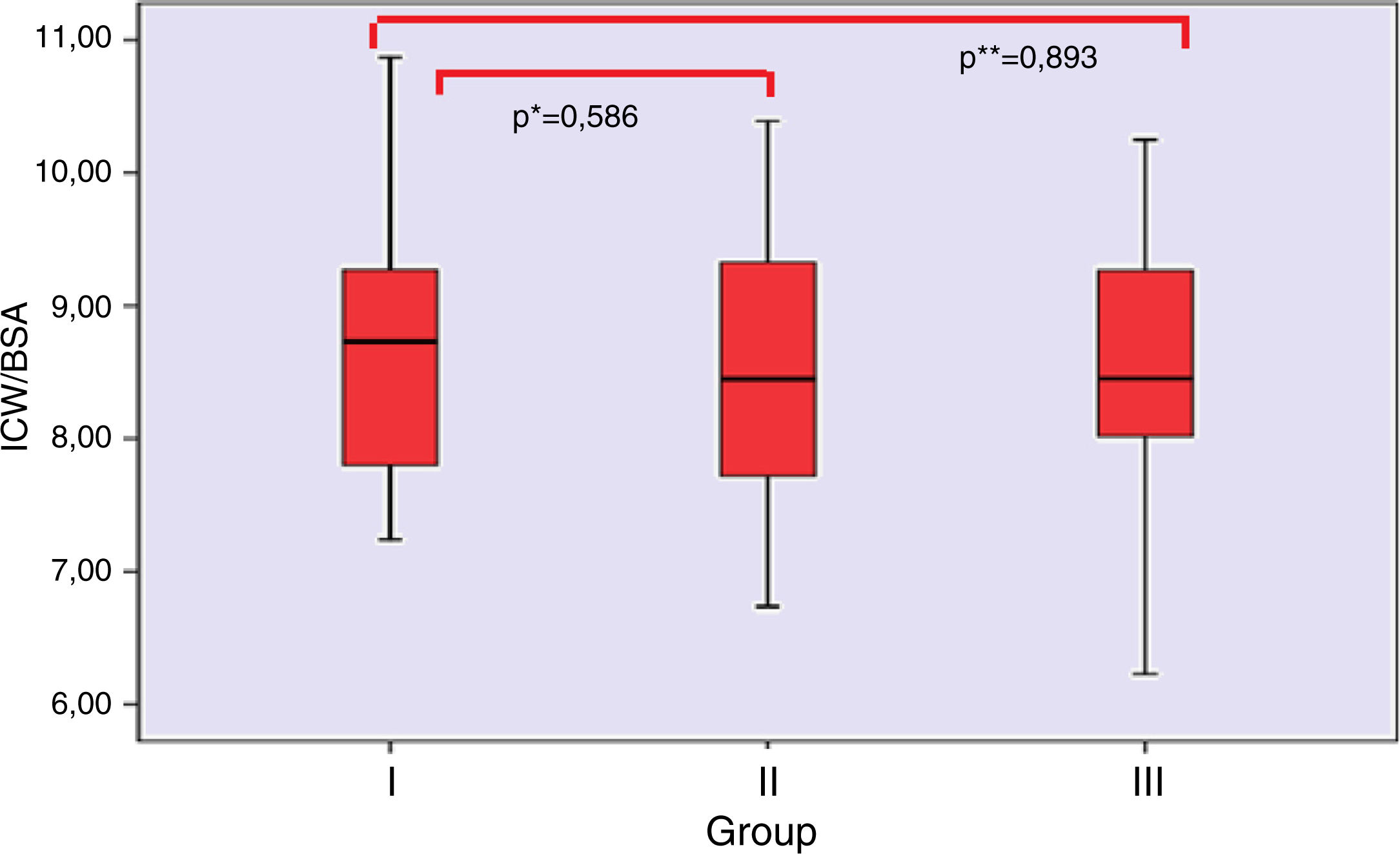
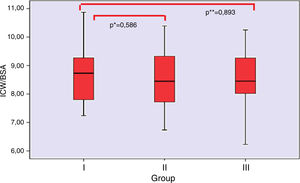
| ICW/BSA | 8.73 (7.24–10.87) | 8.15 (6.7–9.72) | 8.45 (6.2–10.2) | 0.893d | 0.532e |
| ECW/ICW | 1.01 (0.81–1.25) | 0.73 (0.66–0.83) | 0.75 (0.65–0.82) | <0.001d | 0.001e |
| ECW/TBW | 0.5 (0.44–0.56) | 0.42 (0.40–0.45) | 0.42 (0.38–0.45) | <0.001d | 0.001e |
Data are presented as means±standard deviations (x±SD) or as median with range.
Data shown as median values with range, because of the non-normality of data according to the Shapiro–Wilk test.
According to paired t-tests.
p<0.05 was considered significant p value of <0.05 (controls vs. patients).
OH: Absolute fluid overload (AFO).
Rel OH: relative fluid overload (RFO) is defined as the AFO to ECW ratio.
ECW: extracellular water.
ICW: intracellular water.
TBW: total body water.
E/I: ECW/ICW.
BIA assessment was used as another indicator of volume status in the study cohort. A comparison between the groups are presented in Table 2. Over hydration, Relative OH (OH/ECW %), TBW/BSA, ECW/BSA, ECW/ICW, ECW/TBW were higher in group I as compared with the group II and III. There was no significant difference in the ratio of ICW to BSA in the groups (Fig. 2).
No significant correlation was present between BIA parameters, biochemical parameters, NT-proBNP and echocardiographic measurements in group I and group II. Serum NT-proBNP did not correlate with any other variables in group I and group II, but it did correlate with TBW/BSA (r: −0.527 p=0.008), ECW/BSA (r: −0.579 p=0.003) and ECW/ICW (r: −0.452 p=0.023) in group III.
DiscussionIn this study, we observed that neither debut nor relapse nephrotic patients had symptoms of hypovolemia. Also, we found that plasma renin activities, aldosterone levels were not different between groups indicating that edema did not develop as a result of activation of renin–angiotensin–aldosterone (RAA) with a hypovolemic contributing factor. Moreover, our study showed that VCI index, left atrial diameter measured in children with debut or relapse NS were not significantly different than the controls whereas NT-proBNP values were higher than the remission and control groups. Lastly, by using BIA we demostrated that TBW values were increased in association with high ECW but without any change in ICW in patients with NS during attack.
Several methods including clinical signs and symptoms, neurohumoral hormone assays, blood volume measurement with radioactive labeling techniques and IVCD measurements have been utilized in NS patients to assess the hydration status.6,7,22–33 Consistent to several recent studies, during the debute or relapse period, the symptoms of hypovolemia including tachycardia, pallor or abdominal pain were not observed in our study.23,25,28 Plasma renin and aldosterone levels of patients with NS have been investigated widely in previous studies. Elevated renin or aldosterone levels have only been confirmed in about half of studied nephrotic subjects10,22,27,30,31 while some other studies have noted normal or lower values.5,23,31 In our study, the sodium reabsorption was significantly decreased in Group I patients compared to Group II and III. Lower FENa+ values in Group I patients than other two groups, despite the unsignificant difference in terms of plasma renin and aldosterone levels between groups may indicate the role of intrarenal mechanisms in sodium reabsorption in children with INS. Besides renin and aldosterone, we investigated the role of NT-proBNP in determining the volume status of children with NS. NT-proBNP was high in the debut/relapse group during disease. Higher concentration of NT-proBNP in the debut/relapse group suggests the overfill blood volume in these patients.
Previous studies investigating the central volume status by measurements of IVC and left atrial diameter by echocardiography yielded conflicting results in children with INS.23–25,28,34 Recently, Gurgoze et al.,23 reported left atrial diameter and IVCCI values were similar between patients with and without edema and concluded that patients with NS tended to be normovolemic instead of being hypovolemic. Similarly our data show no significant difference between the groups in terms of IVC index and left atrial diameter but a tendency toward an increased IVC inspiratory diameter/BSA ratio was seen in Group I compared with the group III. Our data showed no significant correlation between IVC diameter and NT-proBNP and fluid volume by BIA.
The use of IVC as a marker of central blood volume filling should be researched in a larger patient group. By the way, this method can have some limitations. That means for example, the toddlers are less likely to lie still during the ultrasound tests. It causes most probably undesired biases for measurements. Since the toddlers cry, intra-thoracic and intra-abdominal pressure will obviously increase. Therefore it will show some critical deviances in the IVC diameter measurements. These limitations may underlie the discordance between the current results and the previously reported of echocardiographic IVC measurements24,25,28,34. Point-of-care ultrasound of IVC was not reliable enough to be used as an independent screening method for volume overload to assess younger children.
Although fluid management is based primarily on subjective clinical assessment, clinicians need noninvasive bedside tools to provide a more objective assessment of fluid status. Multifrequency bioimpedance measurement has been claimed to be a reliable noninvasive technology for estimating body water compartments in adults.16,35 Its usage in pediatric patients’ has also been advocated.23,34,36–40 The use of relative overhydration (Rel OH) allows for hydration status comparison between all patient groups including children. Two pediatric abstracts showed the utility of BIA in children older than 2 years of age. One of them validated and established fluid and body composition reference values in 430 healthy children; the other one showed a very similar total body water content estimate by BIA and by the Morgenstern equation, which is the only equation for TBW assessment validated in children on dialysis.38,40
As in the latter study, we observed increased TBW in patients with debut or relapse NS compared to healthy controls in association with increased ECW but without any change in ICW. Previously, Gurgoze et al.23 calculated TBW by measuring resistance and reactance values using a 50kHz BIA device and TBW was found to be markedly higher in patients with edema. Despite the methodological differences between the two studies, patients with NS tended to be hypervolemic instead of being hypovolemic and the increase in TBW is likely associated with increased ECW without any change in ICW. Although BIA was found to be reliable and practical method in pediatric patients on hemodialysis, there is scarce data regarding the usefulness of BIA to evaluate volume status in children with nephrotic syndrome. More recently, Özdemir et al.34 evaluated the clinical findings, echocardiographic measurements (IVC index and IVC collapsibility index) and BIA to determine the volume status of 34 children with NS and controls. The authours concluded that BIS may be a superior technique to echocardiography since the sensitivity and specificity of BIA is relatively higher.
Natriuretic peptides play a key role in regulation of blood pressure and volume homeostasis due to their natriuretic/diuretic and vasodilatory actions.41 Several previous studies have shown the strong relationship between natriuretic peptides and left ventricular hypertrophy, systolic dysfunction but most of the patients included in these studies were prevalent dialysis patients.42,43 We preferred the use of NT-proBNP due to its in vitro stability appropriate for routine clinical use and longer half-life of BNP compared to ANP. We found that NT-proBNP level in Group I was significantly higher compared to Group II and Group III. NT-proBNP levels waned during remission, supporting the volume expansion with NS. In the study by Andersen et al.24 higher ANP and NT-proBNP levels were found in children with aldosterone-suppressed group compared to remission groups suggesting possible overfill blood volume.
To date, however there has been no study evaluating the relationship among NT-proBNP, echocardiography measurements and BIA parameters in children with NS. We did not find any correlation between the parameters of BIA (OH, Rel OH, TBW/BSA, ECW/BSA, ECW/ICW, ECW/TBW) and NT-proBNP in Group I and Group II but positive correlations was observed between ECW/BSA, ECW/ICW and ECW/TBW and NT-proBNP in Group III. These findings may be explained by the renal unresponsiveness to the natriuretic peptides which was previously proposed in NS patients.44,45 Despite their increased levels in NS and other edematous conditions, the underlying renal unresponsiveness to natriuretic peptides include; decreased renal perfusion pressure, RAAS, increased levels of antagonist hormones such as endothelin and AVP, down-regulation in natriuretic peptide receptors, increased breakdown by endopeptidases and release of less active forms.45 The presence of correlation between BIA values and NT-proBNP only in the control group and not in the patients groups may indicate the alteration of the relationship between NT-proBNP and hydration status. The present study has not been specifically designed to explain this observation; however, down-regulation of natriuretic peptide receptors or increased breakdown by neutral endopeptidases may play a role in renal unresponsiveness.
One of this study's weakness is the fact that the subgroup of patients with NS and remission is very small and the lack of long-term, longitudinal-serial measurements. The other limitation was that the sodium intake could not be standardized in these patients studied just at debut or relapse. This may contribute the substantial variation on urinary sodium excretion. It is also important to note that our data represent a single center report.
In conclusion, our results indicate that renin, aldosterone, VCI index, left atrial diameter measured in children with debut or relapse NS were not significantly different than the controls whereas NT-proBNP values were higher than the remission and control groups. Increased TBW values, in association with high ECW but without any change in ICW, indicates the lack of hypovolemia in children with NS. Since BIA provides results immediately, it might be an additional tool for everyday care of difficult patients with NS, whereas natriuretic peptides results are not available in a fast manner most of the time. Further studies are needed to evaluate the utility of BIA at the follow-up children with NS.
Ethical approvalThis study was supported by Ondokuz Mayis University Department of Scientific Research Projects. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee at which the studies were conducted (PYO. TIP.1901.12.019) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interestThe authors have declared that no conflict of interest exists.
We thank those of our patients and clinical colleagues who have supported this research.
We presented this study as a poster presentation in the 47th ESPN Congress in Porto, Portugal, September 18–20, 2014 (p206). This study was supported by Ondokuz Mayis University Department of Scientific Research Projects.