Low levels of thyroid hormones, total triiodothyronine (T3) and free triiodothyronine (FT3) in haemodialysis patients are a marker of malnutrition and inflammation and are predictors of mortality. The aim of this study was to determine the prevalence of malnutrition-inflammation complex syndrome in haemodialysis and its relationship with the thyroid hormones thyrotropin, T3, FT3 and free thyroxine (FT4), as well as to evaluate the prevalence of low FT3 syndrome and its correlation with nutritional and inflammatory markers.
Materials and methodsCross-sectional, analytical and comparative study that enrolled 128 haemodialysis patients: 50.8% females; mean age 45.05±17.01 years; mean time on haemodialysis 45.4±38.8 months; 29.7% diabetics; 79.7% with hypertension. Serum thyroidhormones thyrotropin, T3, FT3 and FT4 concentrations were measured and Malnutrition-Inflammation Score (MIS) was applied to diagnostic.
ResultsMean thyroid hormone values were: thyroid hormones thyrotropin 2.48±1.8mIU/ml (range: 0.015–9.5), T3 1.18±0.39ng/ml (range 0.67–2.64), FT3 5.21±0.96pmol/l (range: 3.47–9.75); FT4 1.35±0.4ng/ml (range: 0.52–2.57). Malnutrition-inflammation complex syndrome prevalence was 53.9%; 11.7% presented low FT3 levels. Serum T3 and FT3 concentrations inversely correlated with Malnutrition-Inflammation Score (MIS), while FT4 correlated positively with Malnutrition-Inflammation Score. In the linear regression analysis, low FT3 was associated with IL-6 (β=0.265, p=.031), C-reactive protein (CRP) (β=−0.313, p=.018) and albumin (β=0.276, p=.002).
ConclusionLow T3 and FT3 levels are correlated with malnutrition and inflammation parameters. Malnutrition-inflammation complex syndrome can affect serum concentrations of thyroid hormones.
La reducción de las hormonas tiroideas, triyodotironina total (T3) y triyodotironina libre (T3L) en pacientes en hemodiálisis, es un marcador de malnutrición e inflamación y son predictores de mortalidad. El objetivo del estudio fue determinar la prevalencia del síndrome complejo de malnutrición e inflamación en hemodiálisis y su asociación con las hormonas tiroideas: tirotropina, T3, T3L y tiroxina libre (T4L); además de evaluar la incidencia del síndrome de T3L y su correlación con marcadores nutricionales e inflamatorios.
Materiales y métodosEstudio transversal, analítico y comparativo, incluyó 128 pacientes en HD, 50,8% mujeres, edad 45,05±17,01 años, 45,4±38,8 meses en hemodiálisis, 29,7% diabéticos y 79,7% hipertensos. Se determinó en suero la concentración de tirotropina, T3, T3L y T4L, se aplicó la encuesta Malnutrition-Inflammation Score para diagnosticar malnutrición e inflamación.
ResultadosLa media de valores de las hormonas tiroideas fueron: tirotropina 2,48±1,8 mUI/mL (rango 0,015–9,5), T3 1,18±0,39ng/mL (0,67-2,64), T3L 5,21±0,96pmol/l (3,47-9,75), T4L 1,35±0,4ng/mL (0,52-2,57). La prevalencia de síndrome complejo de malnutrición e inflamación es 53,9%; un 11,7% mostró T3L baja. Las concentraciones séricas de T3 y T3L correlacionan negativamente con Malnutrition-Inflammation Score y T4L correlaciona positivamente con Malnutrition-Inflammation Score. El análisis de regresión lineal de T3L baja fue asociado con IL-6 (β=0,265 p=0,031), proteína C reactiva (β=-0,313 p=0,018) y albúmina (β=0,276 p=0,002).
ConclusionesBajos niveles de T3 y T3L correlacionan con parámetros de inflamación y nutrición. El síndrome complejo de malnutrición e inflamación puede afectar la concentración sérica de hormonas tiroideas.
Malnutrition is a frequent and serious problem in patients undergoing dialysis. The estimated prevalence is 18–75%.1–3 The etiology is multifactorial and has been called “malnutrition-inflammation complex syndrome (MICS)”4 and currently is also referred as “energy protein wasting syndrome”.5
MICS is a condition in which there is a loss of protein reserves and energy resulting from inflammatory and non-inflammatory causes in patients with chronic kidney disease (CKD). Factors involved are: diet, oxidative stress, acidemia, blood loss by hemodyalizers and through feces, uremic medium and the effect of anabolic hormones.5 There is a survey that is used to make the diagnosis of Malnutrition Inflammation Score (MIS),6 that has been recently validated in Mexico.7
Previous studies have reported that in dialysis patients there is 50% decreased in serum levels of free triiodothyronine (FT3). Low FT3 syndrome is defined as low FT3 with normal thyroid stimulating hormone (TSH) and normal or slightly reduced free thyroxine (FT4) level. This has been correlated with parameters of malnutrition and inflammation.8
In CKD, there is an alteration of the metabolism, distribution, degradation and excretion of thyroid hormones9,10 the most commonly observed is a decrease in total triiodothyronine (T3) concentration.11
The etiology of thyroid disorders in CKD is multifactorial and it is not entirely understood. There are a number of contributing variables: a decreased activity of deiodinase, reduction in the excretion of inorganic iodine, presence of uremic toxins, metabolic acidosis, malnutrition, use of heparin in hemodialysis (HD), advanced age, infection by hepatitis C virus and drugs (amiodarone, steroids, beta-blockers, lithium, rifampin, sunitinib, sorafenib, imatinib, among others).9,12,13
Some studies in CKD patients have found correlations between low concentrations of T3 with high concentrations of inflammatory markers (highly sensitive C reactive protein [hsCRP], interleukin 6 [IL-6]), malnourishment (decrease in serum prealbumin concentration), endothelial dysfunction, deterioration of cardiac function, poor survival and greater mortality from all causes.14–19 Other authors have shown that low serum concentrations of FT3 are associated with an increase in mortality, explained in part by its underlying association with poor nutritional status and inflammation.20,21
The aim of this study was to evaluate the frequency of MICS in patients with HD and its association with changes in serum thyroid hormone concentrations.
MethodsThis is a cross-sectional study, from January 4 to July 30, 2016 at the Regional General Hospital No. 1 of the Mexican Social Security Institute (IMSS), the protocol was approved by the Ethics and Research Committee according to the Declaration of Helsinki regarding the development of research protocols in humans.
The study inclusion criteria were: patients with more than three months on treatment with HD, three sessions per week, of both sexes, between 18 and 79years of age, with no known, or detected, thyroid disease during the study, and a minimum dialysis dose of spKt/V of 1.2 in accordance with the KDOQI guidelines.22 Patients on treatment with drugs that could change the serum concentrations of thyroid hormones, as well as those with chronic diseases, with a positive panel for human immunodeficiency virus, hepatitis B or C virus, or with a documented infection in the previous 6weeks. A clinical history was taken, and a record made of age, gender, previous diagnoses, time on haemodialysis, and medical treatment. Measurements were made of the dry weight and height, and the body mass index (BMI) was calculated.
Blood samples were obtained between 8:00 and 10:00 am, after a 8h fasting before the initiation of the HD session (48h after the last session). Blood measurements included complete blood count, glucose, urea, creatinine, ferritin, transferrin, albumin, cholesterol, triglycerides, CRP, IL-6, TSH, T3, FT3 and FT4.
C reactive protein (CRP) was measured in serum by immunokinetics at the end point (Vitros products, USA) with a dynamic range of 7.0–110.0mg/l.
The measurements of thyroid hormones (TSH, T3, FT3 and FT4) were carried out by chemiluminescence (Vitros 3600 from Johson & Johnson, Ortho Clinical Diagnostics, United Kingdom). The normal reference values were: TSH: 0.465–4.68mUI/ml; T3: 0.97–1.69ng/mL; FT3: 4.26–8.1pmol/l; FT4: 0.78–2.19ng/dl.
An aliquot of serum was frozen −70°C for the subsequent analysis of IL-6, by ELISA (novex® laboratory, by life technologies, Belgium).
The diagnosis of MICS was made by the MIS survey, the following 10 elements were evaluated: dry weight loss, food intake, gastrointestinal symptoms, functional capacity, comorbidity including years on dialysis, decrease in fatty deposits or loss of subcutaneous fat, signs of muscle loss, body mass index, serum albumin, total capacity to transport iron or transferrin. Each of the above elements was given a score of 0 to 3 points. The final sum of the survey was 0–30 points. For MICS diagnosis, the following categories were used: normal (0–2 points), mild malnutrition (3–5 points), moderate malnutrition (6–8 points) and severe malnutrition (greater or equal to 9 points).
Statistical analysisPatients were categorized into patients with and without MICS. The normal distribution was identified by the Kolmogorov–Smirnov test. The data are presented as average±standard deviation or percentages and ranges depending on the variable. The differences between groups, with and without MICS, were analyzed using Student's t test for independent samples and variables with normal distribution. The U Mann Whitney test was used to compare variables that did not have normal distribution and were expressed as median with their respective range. In addition, the Pearson linear correlation coefficient (or Spearman when some variable was ordinal and/or not normally distributed) was used to determine correlations between variables.
A simple linear regression analysis was performed to rule out those confounding variables using thyroid hormone concentrations as a dependent variable and other demographic – biochemical parameters as independent variables.
Two tails p<0.05 was considered significant. The statistical analysis was performed using SPSS® v.20.
ResultsWe included 128 patients, 50.8% women, 29.7% with diabetes mellitus and 79.7% had hypertension. Age 45.05±17.01 years, average time in HD was 45.4±38.8 months, Kt/V: 1.37±0.16 with a percent urea reduction of 65.9±5.7%, 96% of patients were treated with erythropoietin.
The average values of clinical and biochemical variables are shown in Table 1. The average value of IL-6 was 8.60pg/ml raging from 0 to 126.6pg/ml; average ferritin level was 124mg/dl with a range of 8–1610mg/dl. The mean and the range of thyroid hormone concentrations were: TSH 2.48±1.8mUI/mL (0.015–9.5); T3: 1.18±0.39ng/mL (0.67–2.64); FT3: 5.21±0.96pmol/l (3.47–9.75); FT4: 1.35±0.4ng/dl (0.52–2.57).
Main clinical and biochemical characteristics of the 128 chronic hemodialysis patients included in the study.
| Variable | Mean±standard deviation |
|---|---|
| Age (years) | 45.05±17.01 |
| Body mass index (kg/m2) | 23.96±4.98 |
| Systolic blood pressure (mmHg) | 138.48±22.87 |
| Diastolic blood pressure (mmHg) | 75.09±13.27 |
| Time on hemodialysis (months) | 45.4±38.8 |
| Kt/V | 1.37±0.16 |
| Hemoglobin (g/dl) | 10.1±2.19 |
| Glucose (mg/dl) | 111.5±56.5 |
| Urea (mg/dl) | 117.8±40 |
| Creatinine (mg/dl) | 10.2±3 |
| Transferrin (mg/dl) | 233±59 |
| Albumin (g/dl) | 3.89±0.4 |
| Cholesterol (mg/dl) | 137.85±35.2 |
| Triglycerides (mg/dl) | 125.4±72.7 |
| C-reactive protein (mg/l) | 26.4±16.6 |
| Thyroid stimulating hormone (mUI/mL) | 2.48±1.8 |
| Total T3 (ng/mL) | 1.18±0.39 |
| Free T3 (pmol/l) | 5.21±0.96 |
| Free T4 (ng/dl) | 1.35±0.4 |
According to the results of the MIS survey, 53.9% of the patients had MICS of which 55 had hypertension and 25 had were diabetics (DM2); in the group of patients without MICS, 47 were hypertensive and 13 had DM2. These differences between MICS and no MICS were not significant. No significant differences were observed in BMI, blood pressure, Kt/V and URR (Table 2).
Anthropometric parameters in groups with and without MICS.
| Variable | With MICS (n=69) | Without MICS (n=59) |
|---|---|---|
| Age (years) | 47.59±17.9 | 42±15.5 |
| Gender: (M/F) | 29/40 | 25/34 |
| Body mass index (kg/m2) | 23.8±5.55 | 24±4.2 |
| Months in HD (range) | 58.36 (4–240) | 32.85 (3–87) |
| Dry weight (kg) | 58.1±12.6 | 60.5±10.6 |
| Systolic blood pressure (mmHg) | 140.1±24.6 | 136.5±20.6 |
| Diastolic blood pressure (mmHg) | 73.2±13.5 | 77.2±12.7 |
| Kt/V | 1.38±0.18 | 1.37±0.16 |
| URR (%) | 66.1±5.2 | 65.6±5.1 |
Median±standard deviations, range within parentheses.
F: female; Kt/V: dialysis index for quantification of the dialysis dose; K: dialyzer clearance; M: male; t: time; V: volume of distribution of urea; URR: Urea Reduction Ratio.
Most biochemical parameters in MICS and no MICS were significantly different. Differences in CRP should be highlighted (MICS 32.3±20.2 vs. no MICS 12.8±6.3mg/l) (p<0.01). There was also a difference in serum ferritin concentrations ([MICS, 204.8ng/mL vs. no MICS, 42.2ng/mL, p<0.01] and IL-6 [MICS, 11.7 vs. no MICS, 4.8, p<0.01]) (Table 3).
Biochemical differences between patients with and without MICS.
| Variable | With MICS (n=69) | Without MICS (n=59) |
|---|---|---|
| Hemoglobin (g/dl) | 9.4±2.17 | 10.7±2** |
| Glucose (mg/dl) | 120.75±75 | 101.37±42 |
| Urea (mg/dl) | 114.2±38.4 | 122.1±41.7 |
| Creatinine (mg/dl) | 9.2±2.7 | 11.4±3.1** |
| Albumin (g/dl) | 3.72±0.37 | 4.1±0.34** |
| Cholesterol (mg/dl) | 130.9±33.3 | 145.8±36 |
| Triglycerides (mg/dl) | 118±67.5 | 133.9±78.1 |
| C-reactive protein (mg/l) | 32.33±20.2 | 12.8±6.31** |
| IL-6 (pg/ml) | 11.7 (0–126.6) | 4.8 (0–16.4)** |
| Transferrin (mg/dl) | 210.6±59.6 | 259.7±46.1** |
| Ferritin (ng/mL) | 204.8 (8–1610) | 42.2 (8–385)** |
| Thyroid stimulating hormone (mUI/mL) | 2.8±2.2 | 2.36±1.76* |
| Total T3 (ng/mL) | 1.13±0.25 | 1.24±0.35* |
| Free T3 (pmol/l) | 5.1±1 | 5.3±0.84 |
| Free T4 (ng/dl) | 1.48±0.47 | 1.19±0.29** |
Mean±SD, SD: standard deviation; range, within parenthesis.
Regarding the serum concentrations of thyroid hormones, patients with MICS had higher TSH concentrations (MICS 2.8±2.2 vs. no MICS 2.36±1.76) and also had higher FT4 levels (MICS 1.48±0.47 vs. without MICS 1.19±0.29) but the values of T3 were decreased (MICS 1.13±0.25 vs. no MICS 1.24±0.35), p<0.05.
In addition, we found that the dose of erythropoietin was greater in the MICS vs no MICS group (211.24±77.96IU/kg/week vs. 154.8±78.04IU/kg/week) with an index of EPO resistance of 1345.13±570.91 in MICS patients of vs. 968.58±462.65 (p<0.01) in patients without MICS (Table 3).
In MICS patients, 28.9% had mild malnutrition (n=37), 15.6% had moderate malnutrition (n=20), and 9.4% presented severe malnutrition (n=12).
The low FT3 syndrome (<4.26pmol/l), was diagnosed in 15 patients (11.7%), of which 11 had MICS and 4 did not present MICS; the difference was not significant.
Comparison of the 15 patients with low T3 against the 113 patients with normal T3, revealed that those with low T3 were older (55.6±16.3 vs. 43.6±16.6, p=0.01), with higher CRP (41.9±23 vs. 14.5±10.9, p=0.0001), higher ferritin level (246.5±454.24 [range 8–1610] vs. 104.6±189.2 [range 8–1400], p=0.04) and lower albumin level (3.49±0.36 vs. 3.95±0.39, p=0.002).
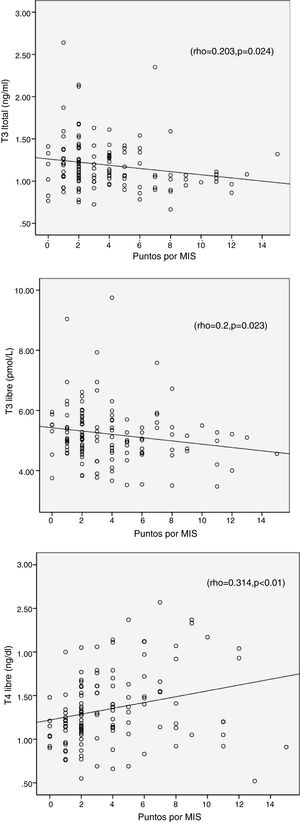
The results from the MIS survey did not show a correlation with TSH (r=0.139, p=0.17), but it was associated positively with FT4 and negatively with T3 and FT3; this is shown in Table 4 and Fig. 1.
Correlation of thyroid profile with demographic and biochemical variables.
| MIS | Age | Creatinine | Albumin | Cholesterol | Triglycerides | PCR | IL-6 | Ferritin | |
|---|---|---|---|---|---|---|---|---|---|
| MIS | 1 | 0.120 | −0.414** | −0.534** | −0.253** | −0.204* | 0.316** | 0.327** | 0.457** |
| TSH | 0.139 | 0.251** | −0.21* | – | – | – | 0.337** | 0.212* | 0.197* |
| T3 | −0.203* | −0.18* | – | 0.305** | – | 0.198* | −0.266* | – | – |
| T3L | −0.2* | −0.25** | 0.195* | 0.262** | – | 0.216* | – | – | −0.196* |
| T4L | 0.314** | 0.277** | −0.327** | −0.279** | −0.224* | −0.236* | 0.218* | 0.313** | 0.226* |
TSH: thyroid stimulating hormone; T3: total triiodothyronine; T3L: free triiodothyronine; T4L: free thyroxine.
Age correlated positively with TSH and FT4 and inversely with T3 and FT3.
Additionally, MIS correlate positively with IL-6, CRP and ferritin, and a negatively with albumin, cholesterol, triglycerides and transferrin (r=−0.417, p<0.001) (Table 4).
Also a significant correlation was observed between low T3 and albumin (r=−0.317, p=0.004), triglycerides (r=0.216, p=0.003), age (r=−0.256, p=0.004), PCR (r=−0.226, p=0.04) and ferritin (r=−0.153, p=0.054).
In the univariate regression analysis, statistical significance was found between TSH and age (β=0.388, p=0.005); T3 and albumin (β=0.47, p=0.004) and, FT4 and IL-6 (β=0.466, p=0.002).
The FT3 did not show statistical significance with any parameter analyzed; however, in patients with low FT3 syndrome (<4.26pmol/l), significance was observed between FT3 and IL-6 (β=0.265, p=0.031), PCR (β=−0.313, p=0.018), and albumin (β=0.276, p=0.002).
DiscussionThere are few studies that associate the results of the MIS survey with thyroid abnormalities in stable chronic HD patients. In the present study the frequency of MICS was 53.9%, which is similar to values previously reported by other authors.3,5 It is important to note that in our study, patients with MICS had higher serum concentrations of TSH and FT4 and lower serum concentrations of T3, creatinine, albumin, cholesterol and triglycerides. In addition it was observed a positive association between MICS and serum concentrations T4L and a negative association of MICS and serum concentrations T3 and T3L.
In addition, we did not find an association of TSH with MICS, this could be attributed to the fact that, in acute phase of critical illness TSH may be normal and in the chronic phase it may be unchanged or be reduced as a sign of recovery.23 On the other hand, we found a positive correlation of TSH with age, CRP, IL-6, ferritin and, unlike other authors, a negative association with creatinine.15
The serum concentrations of the thyroid hormones were similar in men and women. No correlation was observed between of BMI, or hemoglobin with the thyroid profile and markers of inflammation (data not shown).
MICS is negatively associated with T3 and T3L, because T3 is the active form of thyroid hormone and the serum concentrations of T3 in acute or chronic critical illnesses reflect a mechanism of adaptation to the severity of the disease.24
T3L and MIS correlated significantly, but that degree of correlation was not as high as that reported by Yavuz et al. (r=−0.671, p<0.001)24; however the correlation between T3L with age and albumin was similar. We did not find an association between T3L and CRP or IL-6 which is contrary to that reported by other authors,14,15 however we found a significant association with albumin, although with a lower rho value.
Unlike the report by Fernández Reyes et al.,8 we did not find association of T3L with transferrin, but we did find association of low T3L with CRP, this can be explained by the differences in age (45.05±17.01 versus 71±11.7 years) and BMI (23.96±44.98 vs. 26.3±4.8kg/m2) and it can also partially explain the differences observed in the prevalence of low T3L.
We also found an inverse relationship between low T3L and albumin, CRP, ferritin and age, similar to that reported by other authors.14,19 This alteration is frequent in sepsis, cancer, chronic diseases, human immunodeficiency syndrome, myocardial infarction and starvation25 and it was recently proposed as a marker of inflammation in HD patients.24
It is recognized that despite a decrease in T4 concentrations during chronic phase of critical diseases, the concentration of T4L may remain in the normal range, unless the disease is severe and prolonged in which case there is a decrease in TSH as well as in T4 and T4L, being predictor of poor prognosis.23 This is in contrast to our observation of higher levels of FT4 in patients diagnosed with MICS.
Certain drugs compete with the binding to globulin (salicylates, phenytoin, carbamazepine, furosemide, heparin) and this raises the concentration of T4L and reduces T4,23 in our study 20 patients were on furosemide (9 of them in the group of MICS) and one patient used carbamazepine. If these patients are not included the positive association persists (rho=0.271 and p=0.005). Heparin did not influence our results since blood samples were obtained before HD, 48h after the last session.
Other authors have stated that the levels of unsaturated fatty acids may increase in the serum concentration of T4L in non-thyroid disease in CKD, especially when albumin concentrations are low26 this is due to competition for the binding sites of proteins; this is being frequent in severe systemic diseases and malnutrition; Although unsaturated fatty acids were not measured, the mean concentration of albumin in the MICS group was 3.7μg/dl, which is an acceptable level.
The T4L level was increased in the MICS group and also was positively correlated with inflammation; this is contrary to the report by Carrero et al.,15 suggesting that high T4L could be reflect nutritional status and inflammation, which was evidenced by the associations of T4L with other acute phase reactants (PCR, IL-6, ferritin and albumin). In the present study, it was expected to find normal or low T4L levels as seen in patients with non-thyroid disease in CKD,27 this elevation is associated to poor prognosis,23 probably due to an increase in catabolism.
On the other hand, we found 11.7% of patients with low T3, which is in contrast with the 53.1% reported by Fernández-Reyes et al.,8 emphasizing this alteration was more frequent in our MICS patients, and it has been associated to fasting, chronic metabolic acidosis, chronic protein malnutrition which affects the iodothyronine deiodination, and the T3 binding proteins, reducing the peripheral conversion of T4 to T3 and its binding to proteins. This low T3 together with inflammation is associated with a high risk of mortality.11,19,20,27
Some authors claim that inflammatory cytokines such as tumor necrosis factor alpha, interleukin 1 (IL-1) and IL-6 inhibit the expression of the enzyme type 5′-desydase, which is responsible for the peripheral conversion of T4 to T3, and this favors the low level of T3.11,27–29 In our study the CRP is significantly increased in the low T3 syndrome (p=0.044), but IL-6 is not increased (p=0.758) as would have been expected.
The group of patients with MICS had reduced hemoglobin (Hb) levels which can be attributed to the inflammation as illustrated by elevation of CRP, IL-6, ferritin, and the of resistance to erythropoietin similarly to other reports.30,31 There was a negative association between Hb and IL-6 (r=−0.285, p=0.001), CRP (rho=0.314, p=0.005).
Finally, we recognize the limitations in this study. Being a transversal collection of data, it is not possible to establish causality or sequence of events. We were not able to determine iodine, selenium and free fatty acids. Nevertheless, we consider that the results allow valid conclusions.
The findings of this study are directed to the clinician facing the need to perform determinations of nutritional status-inflammation parameters and also thyroid hormones in patients with nephropathy, since they contribute to morbidity and mortality.
ConclusionsThyroid disorders and MICS syndrome are frequent in dialysis, approximately one eighth of these patients showed low T3L syndrome which was correlated with inflammation and malnutrition. Patients with MICS had elevated serum concentrations of T4L and decreased serum concentrations of T3 and T3L. This could have an impact on survival, so these alterations should be investigated in hemodialysis patients.
It will be necessary to perform new prospective studies to evaluate the survival in patients who present an association between MICS and thyroid disorders searching for therapeutic options and evaluating the importance of thyroid hormones measurements as nutritional and prognostic markers.
Conflicts of interestThe authors declare that they have no conflicts of interest.
The authors acknowledge the financial help granted by the Health Research Fund of the IMSS (FIS) number FIS/IMSS/PROT/MD16/1559.
Please cite this article as: Chávez Valencia V, Mejía Rodríguez O, Viveros Sandoval ME, Abraham Bermúdez J, Gutiérrez Castellanos S, Orizaga de la Cruz C, et al. Prevalencia del síndrome complejo de malnutrición e inflamación y su correlación con las hormonas tiroideas en pacientes en hemodiálisis crónica. Nefrologia. 2018;38:57–63.