Patients on haemodialysis (HD) have a high prevalence of 25-OH-vitamin D (25-OH-D)deficiency. Secondary hyperparathyroidismis a common condition in these patients, which is very important to control. 25-OH-D is involved in regulating calcium homeostasis. As such, appropriate levels of this vitamin could help to control bone mineral metabolism.
ObjectiveTo evaluate the effect 25-OH-D repletion in HD patients with 25-OH-D deficiency (<20ng/ml) on the control of secondary hyperparathyroidism and microinflammation status.
Patients and methodsProspective observational study in which stable patients on HD with 25-OH-D deficiency (<20ng/ml) were treated with oral calcifediol 0.266mcg/every 2 weeks for three months. Dialysis characteristics, biochemical parameters and drug doses administered were analysed before and after the correction of the deficiency.
ResultsForty-five stable HD patients with a mean age of 74.08±12.49 years completed treatment. Twenty-seven patients (60%) achieved 25-OH-D levels above 20ng/ml (23 with levels>30ng/ml and 4 between 20 and 30ng/ml). Parathyroid hormone levels decreased in 32 of the 45 patients, 23 of which (51%) achieved a >30% decrease from baseline. In terms of concomitant treatment, we observed a significant reduction in the selective vitamin D receptor activator dose, but no changes in calcimimetic or phosphate binders administration. In terms of malnutrition–inflammation status, a decrease in C-reactive protein was noted, although other microinflammation parameters, such as activated monocytes (CD14+/CD16+ and CD 14++/CD16+) were unchanged. No changes were observed in the levels of FGF-23.
ConclusionsCorrecting 25-OH-D deficiency in HD patients is associated with better secondary hyperparathyroidism control with lower doses of vitamin D analogues, as well as an improvement in inflammatory status. Our results support the recommendation to determine 25-OH-D levels and correct its deficiency in these patients.
El déficit de 25-OH-vitamina D (25-OH-D) es común en los pacientes en hemodiálisis (HD). Por otra parte, es bien conocida la elevada incidencia de hiperparatiroidismo secundario en este grupo de pacientes, y lo importante que es su adecuado control. La 25-OH-D está implicada en la regulación de la homeostasis del calcio, por lo que tener niveles adecuados puede contribuir en el control del metabolismo óseo-mineral.
ObjetivosEvaluar el efecto de la repleción de 25-OH-D en pacientes en HD con déficit vitamínico (niveles<20ng/ml), en el control del hiperparatiroidismo secundario y en el estado de microinflamación.
Pacientes y métodosEstudio observacional, prospectivo en el que se trataron pacientes estables en HD con déficit de 25-OH-D (<20ng/ml), con calcifediol 0,266mcg/15 días vía oral durante 3 meses. Los datos de HD, parámetros bioquímicos y las dosis de fármacos administrados fueron analizados antes y después de la corrección del déficit.
ResultadosUn total de 45 pacientes estables en HD con edad media 74,08±12,49 años completaron el tratamiento. Del total, 27 pacientes (60%) alcanzaron niveles de 25-OH-D>20ng/ml (en 23 fueron>30ng/ml, y 4 entre 20-30ng/ml). Las cifras de hormona paratiroidea descendieron en 32 de los 45 pacientes, alcanzando en 23 (51% de tratados) un descenso>30% respecto al valor basal. En cuanto al tratamiento concomitante, se objetivó un descenso significativo de la dosis de activador selectivo del receptor de vitamina D; sin evidenciarse cambios en la dosis de calcimimético ni de quelantes. Respecto al estado de malnutrición-inflamación, destaca un descenso de la proteína C reactiva, aunque no se modificaron otros parámetros de microinflamación como los monocitos activados (CD14+/CD16+ y CD 14++/CD16+). Tampoco se observaron cambios en los niveles de FGF-23.
ConclusionesLa corrección del déficit de 25-OH-D en pacientes en HD se asocia a un mejor control del hiperparatiroidismo secundario con menores dosis de análogos de vitamina D y a una mejoría en el estado inflamatorio de estos pacientes. Nuestros resultados apoyan la recomendación de determinar niveles de 25-OH-D y corregir el déficit en pacientes en HD.
The hormonal system of native vitamin D (25-OH-D) is linked to the regulation of calcium homeostasis and bone metabolism. 25-OH-D deficiency is very common not only in specific groups of patients, but also in the general population.1 25-OH-D deficiency is highly prevalent in patients with chronic kidney disease in its various stages, and may be found in up to 90% of the population in CKD stage 5D patients (haemodialysis; HD).2,3
Deficiency of 25-OH-D is associated to a greater prevalence of diseases such as cancer4 and cardiovascular disease.5,6 This is possibly explained not only by its relation with bone and mineral metabolism, but also by its pleiotropism.7
Of the pleiotropic effects of 25-OH-D, its role in the immune system and its possible association to chronic inflammation are notable. HD patients present with chronic microinflammation,8 which plays an important role in the elevated morbidity and mortality of these patients. The uraemia-related inflammation can be assessed by the measurement of traditional biochemical parameters (albumin, ferritin or C-reactive protein [CRP]9), however these are not always changed, so it is necessary to use more sensitive methods. Recent studies have shown that the determination of activated monocytes (CD14+/CD16+ and CD 14++/CD16+) in the peripheral blood of patients with chronic kidney disease is more sensitive of inflammation than the conventional methods.10 Determination of activated monocytes may be used to evaluate inflammation in response to treatments or different dialysis techniques.11–13 It is not yet known whether the repletion of 25-OH-D deficiency is capable to modify these inflammatory parameters in HD patients.
It has been shown that 25-OH-D deficiency has a proinflammatory effect on HD patients,14 since anaemia treatment is impaired by inflammation, the correction of 25-OH-D deficiency could lead to an improved response to the anaemia treatment.
Lastly, the relationship between 25-OH-D deficiency and the homeostasis of calcium and phosphorus is clear, but its influence in the control of secondary hyperparathyroidism (SHPT) is not well defined.15 Recent studies have suggested that a repletion of the vitamin could help to improve the control of hyperparathyroidism,16,17 but this effect has not been found in all reports.18,19
The purpose of our study was to evaluate in stable HD patients with 25-OH-D deficiency (levels<20ng/ml) the effect of 25-OH-D repletion on the control SHPT, anaemia and/or the chronic microinflammatory process associated to uraemia.
Patients and methodsProspective, observational single-centre study in stable HD patients. 45 patients were included: 27 men and 18 women with an average age of 74.08±12.49 (ranging from 39 to 85 years), who were in a HD programme for an average of 25±17 months. The aetiologies for the chronic kidney disease included: vascular nephropathy (n=12, 26.6%), chronic glomerulonephritis (n=4, 8.8%), diabetic nephropathy (n=8, 17.7%), polycystic Kidney disease (n=5, 11.1%), urological causes (n=6, 13.3%), systemic diseases (n=2, 3.6%), tubulointerstitial nephropathy (n=3, 6.6%) and a non-affiliated aetiology (n=5, 11.1%).
All patients had 25-OH-D deficiency with serum levels of < 20ng/ml. Each patient received calcifediol (Hidroferol®), a 0.266-mcg ampoule every 2 weeks over the course of 3 months. The patients continued to take the other medications they were receiving prior to the study, which were adjusted at the discretion of the treating doctor depending on the patients’ laboratory or clinical tests; changes in treatments were documented throughout the study. The patients’ diet was not changed.
The patients were dialysed three times per week with a high-flux polysulfone membrane (HF80S; Fresenius Medical Care®, Bad Homburg, Germany). The blood flow rate was 300–400ml/min, and the duration of the dialysis was adjusted on a patient-by-patient basis to maintain a Kt/V of greater than 1.2. All patients were dialysed in the same dialysis unit, using the same dialysate system.
All samples were obtained in lithium heparin tubes and biochemistry test tubes. Haemoglobin levels were measured with an automatic analyser (Abbott Cell-Dyn 4000; Abbott Laboratories®, Abbott Park, IL, USA). The high-sensitivity CRP levels were determined by immunoturbidimetry; the reagents were provided by Abbott Laboratories® (Abbott Park, IL, USA). The normal range of CRP was <5mg/l. The 25-OH-vitamin D levels were determined by the radioimmunoassay (RIA) method (Immunodiagnostic Systems IDS®, Gamma-B kit) in nuclear medicine. The levels of intact parathyroid hormone (PTH) were determined by RIA (Nichols Institute®, The Netherlands).
The determination of activated monocytes (CD14+/CD16+ and CD14++/CD16+) in peripheral blood was carried out after incubating the blood with the monoclonal antibody M5E2 against the CD14 molecule mixed with peridinin-chlorophyll-protein, and with the 3G8 antibody against the CD16 molecule mixed with fluorescein isothiocyanate (FITC). Both antibodies and the appropriate isotype controls were provided by Becton Dickinson® (San Jose, CA, USA). The flow cytometry analysis was conducted with a FACSCalibur flow cytometer (Becton Dickinson®). The number of absolute CD14+ and CD16+ monocytes was obtained using BD TruCOUNT tubes (Becton Dickinson®). To calculate the receptors’ average fluorescence intensity, the flow cytometer was calibrated using 3 BD Calibrite beads (Becton Dickinson®) to adjust the set fluorescence compensation.
In a group of patients, the FGF-23 levels were determined by enzyme-linked immunosorbent assay (ELISA).
The doses of drugs taken by the patients during the study were recorded, and the dose changes during the study period were analysed.
The protocol adhered to the Declaration of Helsinki and was approved by the Independent Ethics Committee of Hospital Universitario Reina Sofía (Córdoba, Spain). Informed consent was obtained from all patients before they were enrolled in the study.
The statistical analysis was conducted using the SPSS statistical software, version 20.0, and the results were expressed as the arithmetic average±standard deviation. Student's t-test was used to analyse the statistical significance of the quantitative parameters for paired data. A p<0.05 was considered as being statistically significant.
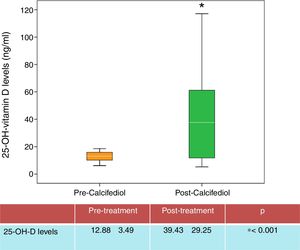
ResultsAfter 3 month treatment with calcifediol, 27 out of the 45 patients (60%) attained the desired 25-OH-D levels (>20ng/ml) (Fig. 1).
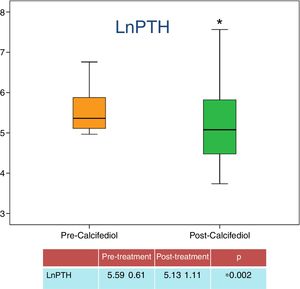
Regarding the effect of 25-OH-D repletion and the control of SHPT, 32 patients (71%) showed a decrease in PTH levels after treatment, with 23 (50% of the total number of patients and 71% of those who reached levels above 20ng/ml) showed a reduction in PTH of more than 30% from the baseline PTH levels. The average PTH levels before and after treatment is seen in Fig. 2.
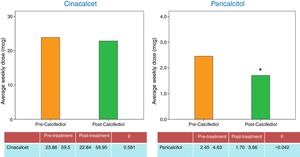
The better control of PTH allowed a reduction in the doses vitamin D analogue (paricalcitol), with no changes made in the dose of calcimimetics throughout the study (Fig. 3). The average calcium levels increased during the study, but it was not statistically significant. Serum phosphorus levels decreased without reaching statistically differences (the average calcium and phosphorus levels are presented in Table 1). The decrease in phosphataemia was attained without changing the doses of phosphate binders. A greater decrease in serum phosphate was noted in patients whose dose of vitamin D analogues was reduced during the study. However, there was no significant correlation between the decrease in phosphataemia and the reduction in the dose of vitamin D analogues. This greater control of phosphorus levels may be attributed to a better control of SHPT.
Main findings determined before and after the correction of 25-OH-D deficiency.
| n=45 | Pre-calcifediol | Post-calcifediol | p |
|---|---|---|---|
| 25-OH-D (ng/ml) | 12.88±3.49 | 39.43±29.25 | 0.001 |
| Haemoglobin (g/dl) | 11.36±1.5 | 11.2±1.56 | 0.145 |
| LnFerritin | 5.96±0.79 | 6.1±0.75 | 0.093 |
| Darbepoetin (mcg/week) | 24.33±18.51 | 23.88±21.12 | 0.85 |
| Iron (mg/week) | 78.88±83.24 | 62.22±74.92 | 0.006 |
| Calcium (mg/dl) | 9.05±0.86 | 9.27±0.79 | 0.058 |
| Phosphorus (mg/dl) | 4.45±1.28 | 4.12±1.36 | 0.161 |
| Cinacalcet mg/week | 23.86±59.5 | 22.84±59.95 | 0.581 |
| Paricalcitol (mcg/week) | 2.45±4.63 | 1.70±3.66 | 0.042 |
| LnPTH | 5.59±0.61 | 5.13±1.11 | 0.002 |
| Albumin (g/dl) | 3.48±0.28 | 3.45±0.48 | 0.870 |
| LnCRP | 1.6±0.88 | 0.16±1.2 | 0.001 |
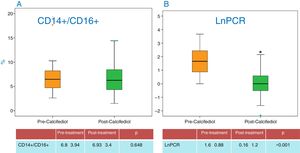
| CD14+/CD16+ (%) | 6.8±3.94 | 6.93±3.4 | 0.648 |
| CD14++/CD16+ (%) | 3.59±2.55 | 4.16±2.88 | 0.458 |
CRP: C-reactive protein; PTH: parathyroid hormone; 25-OH-D: 25-OH-vitamin D.
Soluble levels of FGF-23, a molecule related to phosphorus homeostasis and SHPT control, were determined from the findings of our study regarding greater control of SHPT. These levels were much higher in HD patients than in healthy patients. In HD patients, the average FGF-23 levels did not change before and after the 25-OH-D repletion (pre-treatment average of 432.157361 vs. 541.852406 post-treatment, p=0.47). The same results had been observed in earlier studies.20
The results obtained in this study regarding chronic microinflammation in HD patients were determined using conventional biochemical parameters such as CRP, albumin and ferritin, and also using parameters obtained through flow cytometry, such as the percentage of activated monocytes (CD14+/CD16+ and CD14++/CD16+).
The level of CRP significantly decreased after vitamin D replacement (Fig. 4B), indicating less patient inflammation. No changes were noted in other parameters related to inflammation, such as albumin or ferritin. The data are presented in Table 1.
The percentage of activated monocytes (CD14+/CD16+ and CD14++/CD16+) did not change during the study (Fig. 4A).
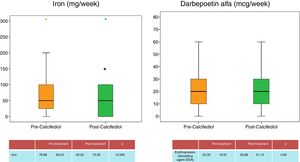
Regarding anaemia, haemoglobin levels remained stable throughout the study (Table 1), and the doses the darbepoetin were maintained (Fig. 5), however the weekly dose of iron dose was reduced (Fig. 5), which may be related to the improvement in patient inflammation as determined by the significant decrease in CRP.
The drugs taken by the patients and the doses thereof were recorded during the study, with the changes being registered down.
DiscussionAfter three months of treatment with native vitamin D (calcifediol), 60% of the patients had adequate 25-OH-D levels (>20ng/ml). This was achieved without any drug-related adverse effect during the treatment period and with a high tolerance to the treatment.
In this group of patients, the correction of 25-OH-D deficiency was associated with a better control of SHPT, which was accompanied by a lower requirement for vitamin D analogues (paricalcitol). The doses of other commonly used drugs in SHPT treatment, such as calcimimetics or phosphate binders, was not changed during the study. These data have already been published previously,16,17 but with different treatment regimens. As with earlier studies,20 no significant decrease in FGF-23 plasma levels were seen in our patients.
Regarding chronic microinflammation, it should be noted that there was a significant decrease in CRP levels after treatment, which is a parameter correlated to microinflammation in HD patients.9 This decrease in CRP is therefore attributed to a decrease of inflammation. Parameters such as ferritin and albumin, which are also linked to microinflammation, did not change during the study.
Regarding the correction of anaemia, no differences were noted in haemoglobin levels before and after repletion with 25-OH-D, and no changes were seen in the doses of erythropoiesis-stimulating agents. However, the weekly requirements for intravenous iron were decreased, which was possibly related to the reduced inflammation after repletion of the 25-OH-D deficiency. These data are similar to those found in previous studies,14 which relate 25-OH-D deficiency to inflammation and, therefore, to a worse response to anaemia treatment.
Our study has limitations such as the moderate response rate (60%) at the end of the study which may be the main limiting factor. This rate of response to the treatment is lower than in other published studies, even though treatment completion was guaranteed because it was administered at the end of dialysis sessions. This relatively low response rate is possibly related to the average age of the patients (74 years), which is higher than that of other published studies. Alternatively, it may be related to treatment duration; perhaps to obtain an optimal drug response rate, the duration of treatment should have been longer.
Nevertheless, and regardless of the study's limitations, the results obtained in terms of SHPT control and inflammation in this group of patients are encouraging, because both processes are closely linked to the high morbidity and mortality rates in this group of patients. The correction of 25-OH-D deficiency in HD patients is associated with a greater control of SHPT using lower doses of vitamin D analogues, and with an improvement in the inflammation experienced by these patients. Our results therefore support the current recommendations of good clinical practice guidelines for determining 25-OH-D levels and for correcting the deficiency in HD patients.
FundingThis study was funded by Amgen® España S.A. Amgen had no role in the study design, the collection, analysis or interpretation of the data, the drafting of the report, or the decision to publish the study results.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Ojeda López R, Esquivias de Motta E, Carmona A, García Montemayor V, Berdud I, Martín Malo A, et al. La corrección del déficit de 25-OH-vitamina D mejora el control del hiperparatiroidismo secundario y el estado inflamatorio de pacientes estables en hemodiálisis. Nefrologia. 2018;38:41–47.